|
New stars and planets form in dense interstellar molecular clouds (Figure 1). These clouds are sufficiently dense to screen out starlight from outside the cloud, so that the dust grains that are present in these dense clouds can get as cold as 10 K (-263 °C). At these temperatures, most gas-phase species other than hydrogen, helium, and neon will freeze out onto these cold dust grains, much as the water vapor in your breath freezes onto a cold window. As a result, most of the grains in dense clouds are coated with a mantle of ice that contains a variety of molecules.

Figure 1: Dense clouds like in the Pillars of Creation in the Eagle
Nebula (M16) are the birth sites of new stars and planetary systems.
The composition of these ices varies with local dense cloud conditions, but infrared spectra taken from these clouds indicates that the ices are generally dominated by H2O, the same material in ice cube trays in our refrigerators. However, these ices often also contain a host of other simple molecules, including methanol (CH3OH), carbon monoxide (CO), carbon dioxide (CO2), ammonia (NH3), methane (CH4), formaldehyde (H2CO), and so on.
While these ices exist in interstellar dense clouds, they are exposed to various forms of high-energy radiation in the form of cosmic rays (high-speed particles) and ultraviolet (UV) photons (see Figure 2). This radiation can break many of the chemical bonds of the molecules in the ices, resulting in the production of ions, radicals, and other highly reactive chemical species. These can react within the ice, either immediately or later when the ice is warmed (for example, by a star forming nearby), to result in the production of new chemical compounds that did not previously exist in the ice. Similar processes would also have occurred to the ices in the protosolar disk from which our Solar System formed, and are currently occurring in exposed ices in the Solar System, for example, on the surfaces of comets and icy bodies in the outer Solar System.
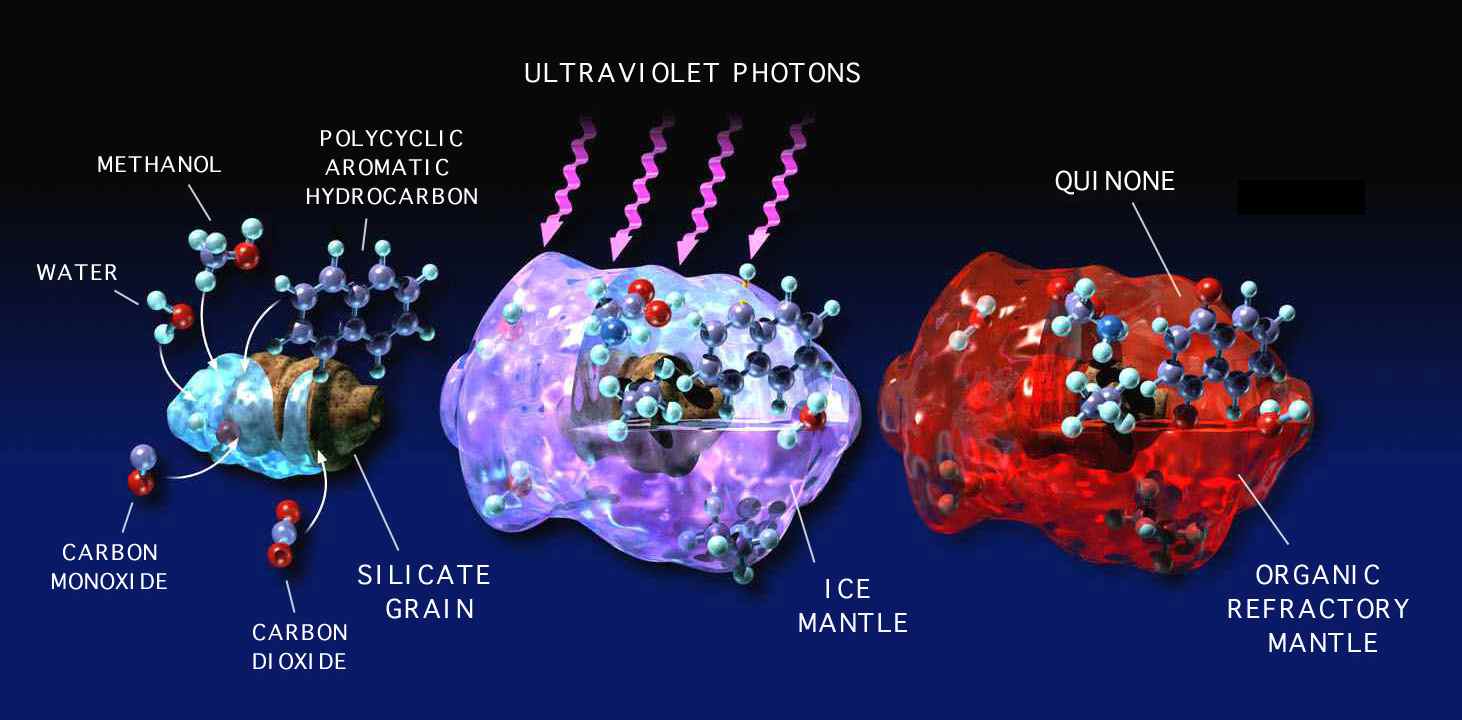
Figure 2: The temperatures in interstellar dense clouds (10–100 K) are low enough
that most gas-phase species freeze into ice mantles on dust grains. High-energy radiation
can convert some of these icy materials into more complex organic compounds.
Simulating Cold Space Environments in the Laboratory
We can simulate these chemical processes in our laboratory by mimicking the conditions of these astrophysical environments, namely, low temperatures, high vacuum, and high radiation. We do this by using cryovacuum systems of the type shown in the Figures 3 and 4. Each of these units consists of a vacuum system capable of attaining pressures as low as 10-8 millibars in the sample chamber. Suspended in the sample chamber of each apparatus is a sample head with a substrate (aluminum foil, IR-transparent window, etc.) that can be cooled to temperatures as low as 5–20 K (depending on the system). Mixed gases can be sprayed onto the cold head to produce ices of the desired composition. These ices are then simultaneously irradiated with high-energy photons from a variety of different lamps or by charged particles, like electrons. We frequently use hydrogen (H2) plasma lamps that produce UV photons in the Lyman-α range (10.2 eV or 121.6 nm).
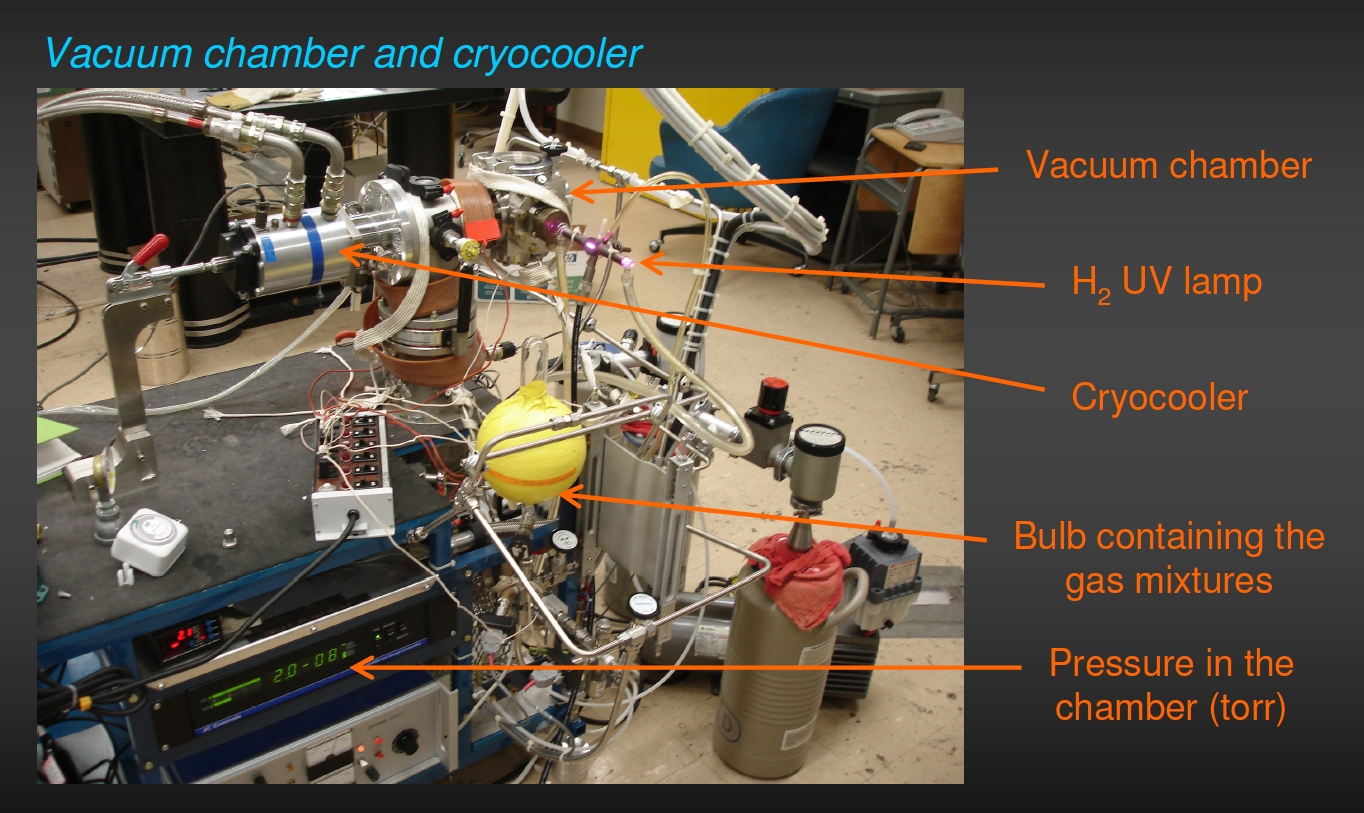
Figure 3: An example of one of the cryovacuum systems in
which we condense and simultaneously irradiate astrophysical ice analogs.
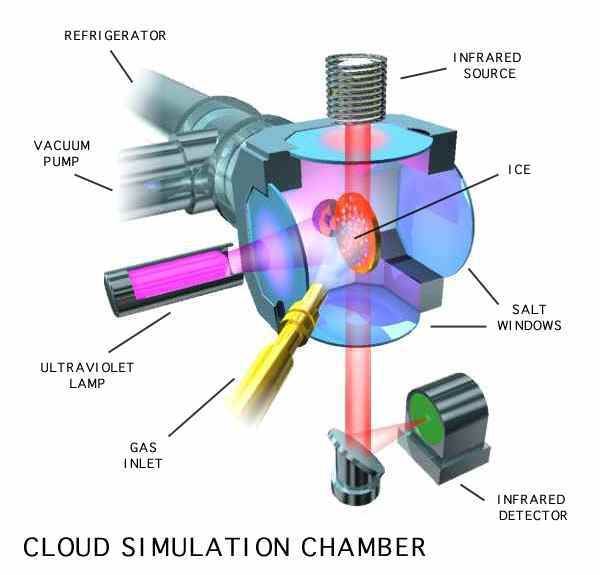
Figure 4: A schematic of a sample vacuum chamber.
The sample chambers of these systems are equipped with windows that allow us to use UV, visible, and IR spectrometers to study the ices before, during, and after irradiation, and during any subsequent warming and sublimation of the ices.
When these ices are warmed after irradiation, the original volatile materials, as well as new volatile species produced during irradiation such as ethanol (CH3CH2OH), formamide (NH2CHO), and acetamide (CH3CONH2), sublime away into the gas phase, leaving behind an organic residue on the substrate (Figure 5). This residue consists of a host of more chemical species created during the irradiation process and the warm-up phase that are significantly more complex than the starting ices. These new materials can then be removed from the system and examined using a host of analytical techniques, such as IR spectroscopy, gas chromatography coupled to mass spectrometry (GC-MS), high-performance liquid chromatography (HPLC), X-ray absorption near-edge structure (XANES) spectroscopy, and two-step laser mass spectrometry (L2MS).
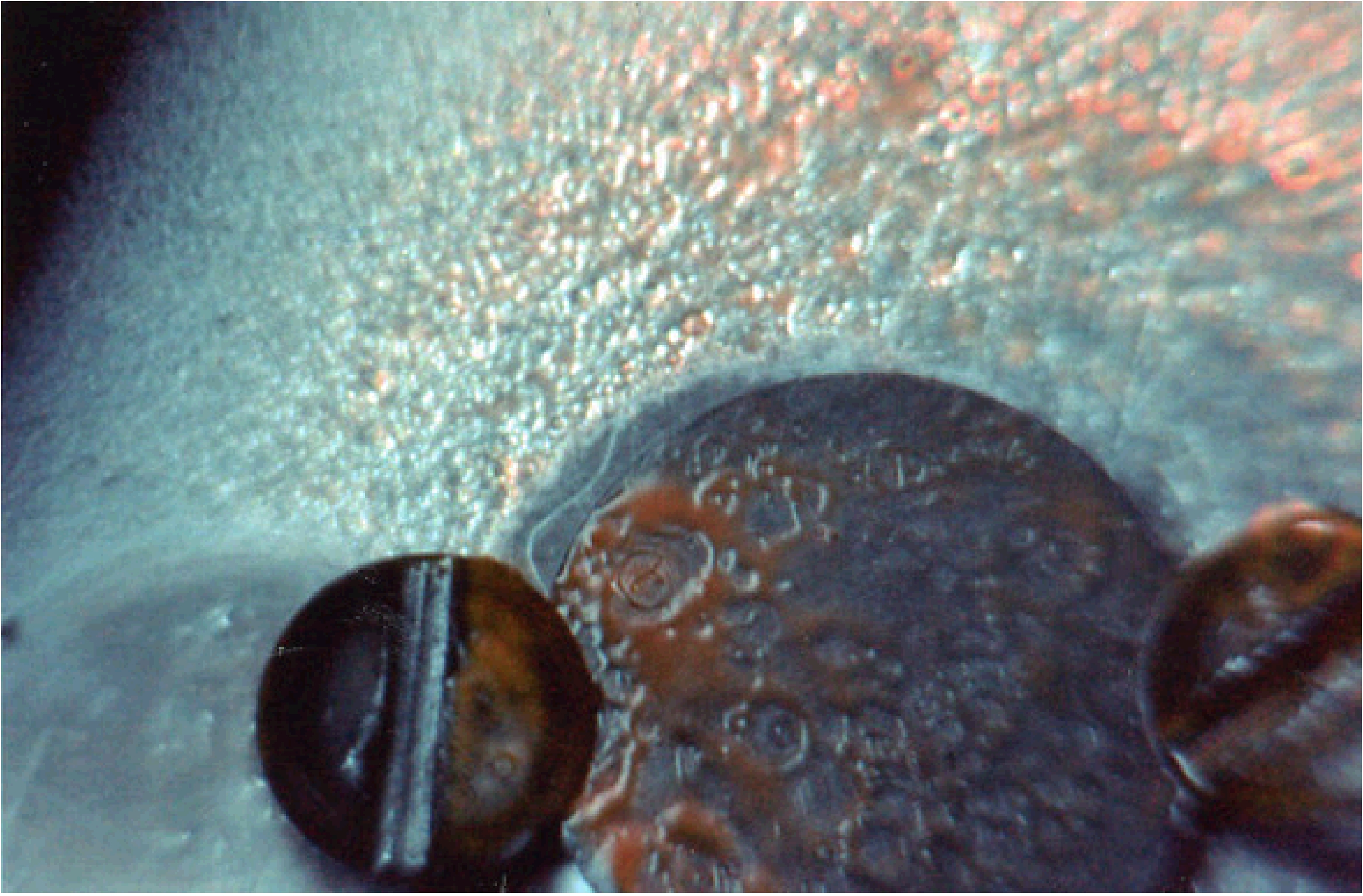
Figure 5: An example of an organic residue resulting from
the UV irradiation of a simple astrophysical ice analog containing H2O, CH3OH, CO, and NH3.
The Nature of Organic Materials Produced by the Irradiation of Astrophysically Relevant Mixed Molecular Ices
The composition of the organic residues produced when astrophysically relevant mixed molecular ices are exposed to high-energy radiation is not yet fully understood. Even in simple ices that contain only H2O, CH3OH, and NH3, the mass spectra of the residues produced upon irradiation show the presence of hundreds to thousands of new compounds.
A significant fraction of these more complex compounds have been identified in the residues, including a number of species that have potential importance for astrobiology and the issue of the origin of life. Some of the compounds of astrobiological significance that have been identified in our ice photolysis residues include:
-
Amino acids (the building blocks of proteins)
-
Sugar derivatives (some of the building blocks of RNA and DNA, as well as energy storage)
-
Amphiphiles (the building blocks of membranes)
-
Polyoxytheylene (POM) and related compounds
-
Hexamethylenetetramine (HMT; precursor reactant to make amino acids, cyanides, etc.)
In addition, if polycyclic aromatic hydrocarbons (PAHs) and related N-containing aromatic compounds are present in the ices, we also see the formation of several classes of cyclic aromatic compounds such as:
-
Quinones (oxidized aromatic molecules used for a host of processes in biochemistry)
-
Hn-PAHs (PAHs with extra peripheral H atoms)
-
Nucleobases (some of the building blocks of RNA and DNA)
Click on the links to learn more about each of these specific types of compounds we find in our residues.
Formation of Meteoritic Insoluble Organic Material
If organic residues are themselves exposed to high-energy radiation such as UV or X-ray photons, the materials are further chemically altered. These chemical changes have so far been poorly studied, but exposure of residues to X-ray photons when they were analyzed for XANES spectroscopy showed that aliphatic carbon is converted into aromatic carbon, as shown in Figure 6 below. This process is associated with the elimination of H atoms from the residue and makes the material look more like meteoritic insoluble organic matter (IOM).
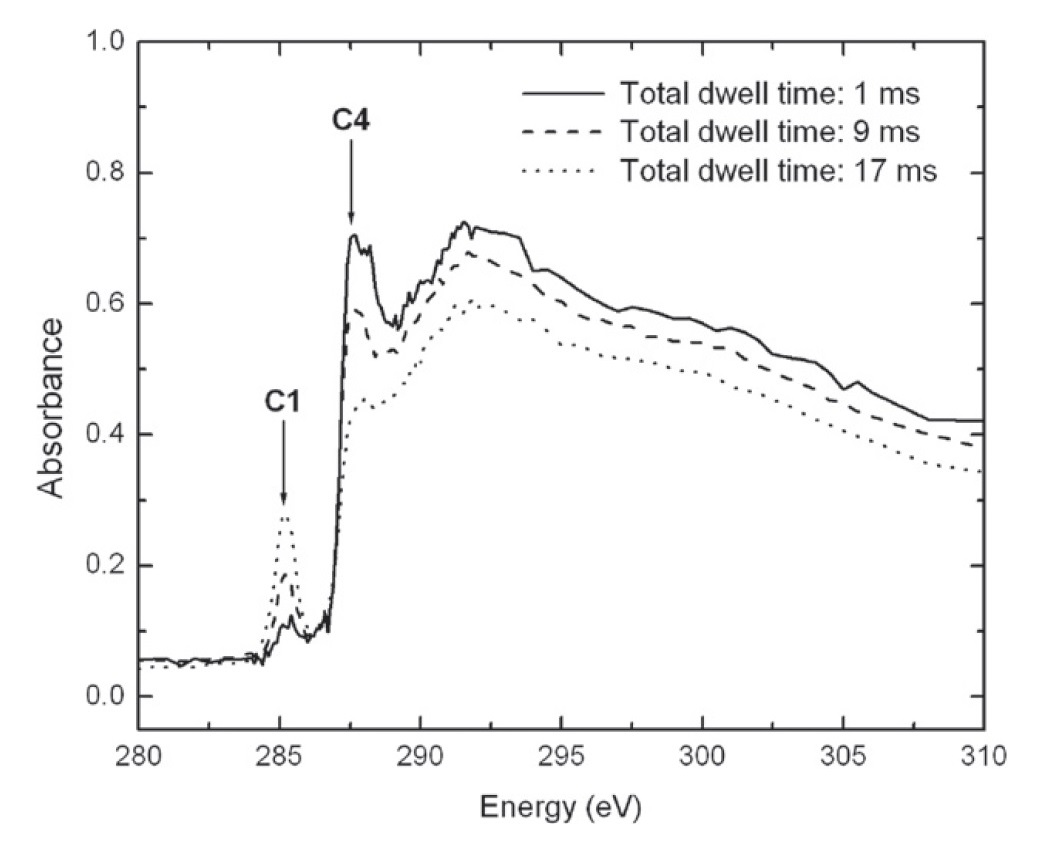
Figure 6. When organic residues are exposed to X-ray radiation, the abundance of aliphatic carbon (band C4) decreases while that of aromatic carbon (C1) increases.
This irradiation-induced chemical alteration of residues may have important implications for the compositional evolution of organic materials in the Solar System. Indeed, organic compounds on dust grains are believed to have received large radiation doses as they mixed in the protosolar nebula (see Figure 7).
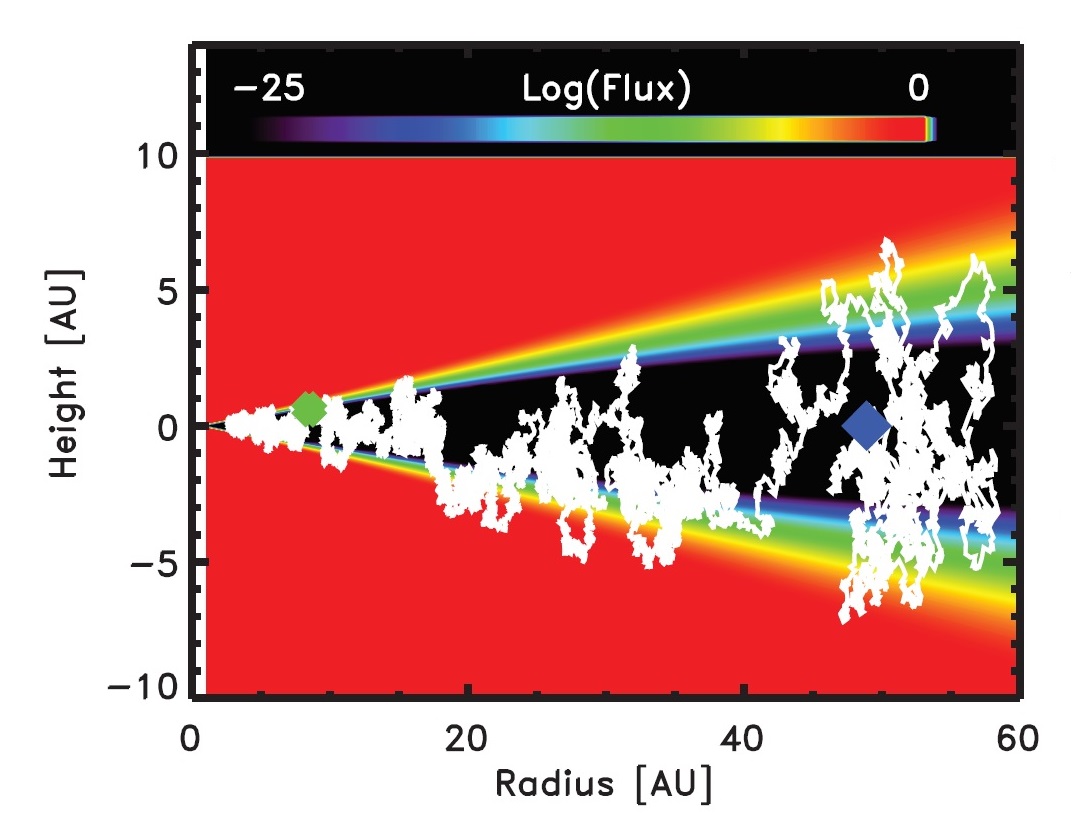
Figure 7. Simulation showing the path of a 1-μm ice-coated
grain in a protoplanetary disk carried by turbulence from optically thick
regions (black area) to surface layers (blue, green, and yellow areas) that see
orders of magnitude increases in the radiation flux. The blue and green
diamonds indicate the starting and ending points of the path, respectively.
The Astrobiological Significance of Organic Residues Produced by the Irradiation of Ices
The production of prebiotic molecules in the interstellar medium and the protosolar nebula from which our Solar System formed is of little consequence to the origin of, and search for, life unless these molecules can be delivered intact to habitable planets where they can potential play some role in getting life started. This requires that these molecules survive the transition from the dense cloud into the protostellar nebula of a forming star, followed by subsequent incorporation into planetesimals that deliver these materials to a planetary surface.
The presence of excess deuterium (heavy hydrogen), an indicator of the presence of extraterrestrial materials, in certain organic molecules in meteorites and cometary and asteroidal dust particles demonstrate that some interstellar/protostellar organic species do, in fact, survive planetary accretion, and are delivered to the Earth’s surface. Indeed, meteorites are known to contain a variety of organic compounds of astrobiological importance that include amino acids, sugar derivatives, nucleobases, and amphiphiles, and that they are currently delivering these extraterrestrial organics to the Earth (Figure 8) .
Figure 8: Extraterrestrial materials, including organics, some of which have
an interstellar heritage, are delivered to the surface of planets via meteorites and cosmic dust.
The observation that the irradiation of astrophysically relevant ices results in the abiotic (non-biological) production of a number of compounds of biological significance, many of which are seen in meteorites, has important astrobiological implications. First, it suggests that the early Earth was almost certainly seeded with these types of compounds, and that these materials would likely have been available to play key roles in the origin of life on our planet. Second, since the irradiation of ices is a process that is expected to occur universally in all interstellar dense molecular clouds, where new stars and planets form, these types of materials should be present at the birth of most planetary systems. Thus, insofar as these materials may have played an important role in the formation of life on the Earth, they would be expected to be present to play a similar role on any newly formed planets that have conditions conducive to the formation and evolution of life. The ubiquity of these ingredients for life suggests that life may not be uncommon elsewhere in the universe.
For more detailed information and reviews on our laboratory work on interstellar and cometary ice analogs, see:
Sandford, S. A., Nuevo, M., Bera, P. P., Lee, T. J. (2020). Prebiotic Astrochemistry and the Formation of Molecules of Astrobiological Interest in Interstellar Clouds and Protostellar Disks. Chem. Rev. 120, 4616–4659.
Materese, C. K., Nuevo, M., Sandford, S. A., Bera, P. P., Lee, T. J. (2020). The Production and Potential Detection of Hexamethylenetetramine-Methanol and Other Hexamethylenetetramine Derivatives in Space. Astrobiology 20, 601–616.
Bera, P. P., Sandford, S. A., Lee, T. J., & Nuevo, M. (2019). The Calculated Infrared Spectra of Functionalized Hexamethylenetetramine (HMT) Molecules. Astrophys. J. 884, 64 (16 pp).
Nuevo, M., Cooper, G., & Sandford, S. A. (2018). Deoxyribose and Deoxysugar Derivatives from Photoprocessed Astrophysical Ice Analogues and Comparison to Meteorites. Nat. Commun. 9, 5276 (10 pp).
Materese, C. K., Nuevo, M., McDowell, B. L., Buffo, C. E., Sandford, S. A. (2018). The Photochemistry of Purine in Ice Analogs Relevant to Dense Interstellar Clouds. Astrophys. J. 864, 44 (6 pp).
Cooper, G., Rios, A. C., & Nuevo, M. (2018). Monosaccharides and their Derivatives in Carbonaceous Meteorites: A Scenario for their Synthesis and Onset of Enantiomeric Excesses. Life 8, 36 (29 pp).
Materese, C. K., Nuevo, M., & Sandford, S. A. (2017). The Formation of Nucleobases from the Ultraviolet Photo-Irradiation of Purine in Simple Astrophysical Ice Analogs. Astrobiology 17, 761–770.
Materese, C. K., Cruikshank, D. P., Sandford, S. A., Imanaka, H., & Nuevo, M. (2015). Ice Chemistry on Outer Solar System Bodies: Electron Radiolysis of N2-, CH4-, and CO-containing ices. Astrophys. J. 812, 150 (9 pp).
Materese, C. K., Nuevo, M., & Sandford, S. A. (2015). N- and O-Heterocycles from the Irradiation of Benzene and Naphthalene in H2O/NH3-Containing Ices. Astrophys. J. 800, 116 (8 pp).
Nuevo, M., Materese, C. K., & Sandford, S. A. (2014). The Photochemistry of Pyrimidine in Realistic Astrophysical Ices and the Production of Nucleobases. Astrophys. J. 793, 125 (7 pp).
Materese, C. K., Cruikshank, D. P., Sandford, S. A., Imanaka, H., Nuevo, M., & White, D. W. (2014). Ice Chemistry on Outer Solar System Bodies: Carboxylic Acids, Nitriles, and Urea Detected in Refractory Residues Produced from the UV Photolysis of N2:CH4:CO-Containing Ices. Astrophys. J. 788, 111 (11 pp).
Materese, C. K., Nuevo, M., Bera, P. P., Lee, T. J., & Sandford, S. A. (2013). Thymine and Other Prebiotic Molecules Produced from the Ultraviolet Photo-Irradiation of Pyrimidine in Simple Astrophysical Ice Analogs. Astrobiology 13, 948–962.
Nuevo, M., Milam, S. N., & Sandford, S. A. (2012). Nucleobases and Prebiotic Molecules in Organic Residues Produced from the Ultraviolet Photo-Irradiation of Pyrimidine in NH3 and H2O+NH3 Ices. Astrobiology 12, 295–314.
Ciesla, F. J. & Sandford, S. A. (2012). Organic Synthesis via Irradiation and Warming of Ice Grains in the Solar Nebula. Science 336, 452–454.
Nuevo, M., Milam, S. N., Sandford, S. A., De Gregorio, B. T., Cody, G. D., & Kilcoyne, A. L. D. (2011). XANES Analysis of Organic Residues Produced from the UV Irradiation of Astrophysical Ice Analogs. Adv. Space Res. 48, 1126–1135.
Nuevo, M., Milam, S. N., Sandford, S. A., Elsila, J. E., & Dworkin, J. P. (2009). Formation of Uracil from the UV Photo-Irradiation of Pyrimidine in Pure H2O Ices. Astrobiology 9, 683–695.
Ashbourn, S. F. M., Elsila, J. E., Dworkin, J. P., Bernstein, M. P., Sandford, S. A., & Allamandola, L. J. (2007). Ultraviolet Photolysis of Anthracene in H2O Interstellar Ice Analogs: Potential Connection to Meteoritic Organics. Meteorit. Planet. Sci. 42, 2035–2041.
Elsila, J. E., Dworkin, J. P., Bernstein, M. P., & Sandford, S. A. (2007). Mechanisms of Amino Acid Formation in Interstellar Ice Analogs. Astrophys. J. 660, 911–918.
Elsila, J. E., Hammond, M. R., Bernstein, M. P., Sandford, S. A., & Zare, R. N. (2006). UV Photolysis of Quinoline in Interstellar Ice Analogs. Meteorit. Planet. Sci. 41, 785–796.
Sandford, S. A. (2005). Organic Synthesis in Space. In Astrophysics of Life, Space Telescope Institute Symposium Series, Vol. 16, M. Livio, N. Reid, & W. Sparks, eds., (Cambridge Univ. Press: Cambridge), pp. 54–66 [ISBN: 0-521-82490-7]
Bernstein, M. P., Ashbourn, S., Sandford, S. A., & Allamandola, L. J. (2004). The Lifetimes of Nitriles (–C≡N) and Acids (–COOH) During Ultraviolet Photolysis and Their Survival in Space. Astrophys. J. 601, 365–370.
Bernstein, M. P., Moore, M. H., Elsila, J. E., Sandford, S. A., Allamandola, L. J., & Zare, R. N. (2003). Side group Addition to the PAH Coronene by Proton Irradiation in Cosmic Ice Analogs. Astrophys. J. 582, L25–L29.
Deamer, D., Dworkin, J. P., Sandford, S. A., Bernstein, M. P., & Allamandola, L. J. (2002). The First Cell Membranes. Astrobiology 2, 371–381.
Bernstein, M. P., Elsila, J. E., Dworkin, J. P., Sandford, S. A., Allamandola, L. J., & Zare, R. N. (2002). Side Group Addition to The Polycyclic Aromatic Hydrocarbon Coronene by Ultraviolet Photolysis in Cosmic Ice Analogs. Astrophys. J. 576, 1115–1120.
Bernstein, M. P., Dworkin, J. P., Sandford, S. A., & Allamandola, L. J. (2001). Ultraviolet Irradiation of Naphthalene in H2O Ice: Implications for Meteorites and Biogenesis. Meteorit. Planet. Sci. 36, 351–358.
Bernstein, M. P., Sandford, S. A., Allamandola, L. J., Gillette, J. S., Clemett, S. J., & Zare, R. N. (1999). UV Irradiation of Polycyclic Aromatic Hydrocarbons in Ices: Production of Alcohols, Quinones, and Ethers. Science 283, 1135–1138.
Bernstein, M. P., Sandford, S. A., & Allamandola, L. J. (1996). Hydrogenated Polycyclic Aromatic Hydrocarbons (Hn-PAHs) and the 2940 and 2850 Wavenumber (3.40 and 3.51 Micron) Infrared Emission Features. Astrophys. J. 472, L127–L130.
Bernstein, M. P., Sandford, S. A., Allamandola, L. J., Chang, S., & Scharberg, M. A. (1995). Organic Compounds Produced by Photolysis of Realistic Interstellar and Cometary Ice Analogs Containing Methanol. Astrophys. J. 454, 327–344.
Bernstein, M. P., Sandford, S. A., Allamandola, L. J., & Chang, S. (1994). Infrared Spectrum of Matrix-Isolated Hexamethylenetetramine in Ar and H2O at Cryogenic Temperatures. J. Phys. Chem. 98, 12206–12210.
Schutte, W. A., Allamandola, L. J., & Sandford, S. A. (1993). Organic Molecule Production in Cometary Nuclei and Interstellar Ices by Thermal Formaldehyde Reactions. Icarus 104, 118–137.
Schutte, W. A., Allamandola, L. J., & Sandford, S. A. (1993). Formaldehyde and Organic Molecule Production in Astrophysical Ices at Cryogenic Temperatures. Science 259, 1143–1145.
Allamandola, L. J., Sandford, S. A., & Valero, G. (1988). Photochemical and thermal evolution of interstellar/pre-cometary ice analogs. Icarus 76, 225–252.
Sandford, S. A. (1998). Organic Chemistry: From the Interstellar Medium to the Solar System. In ORIGINS, Astron. Soc. Pacific Conf. Series, Vol. 148, Proceedings of the International Conference, Estes Park, Colorado, 19–23 May 1997, C. E. Woodward, J. M. Shull, & H. A. Thronson, Jr. (eds.), (ASP: San Francisco), pp. 392–414.
Sandford, S. A., Allamandola, L. J., & Bernstein, M. P. (1997). The Composition and Ultraviolet and Thermal Processing of Interstellar Ices. In From Star Dust to Planetesimals, Astron. Soc. Pac. Conf. Ser., Vol. 122, Y. J. Pendleton & A. G. G. M. Tielens (eds.), (ASP: San Francisco), pp. 201–213.
Bernstein, M. P., Allamandola, L. J., & Sandford, S. A. (1997). Complex Organics in Laboratory Simulations of Interstellar/Cometary Ices. In Complex Organics in Space, 31st COSPAR Scientific Assembly, July 1996, Birmingham, UK, Advances in Space Research 19, 991–998.
Allamandola, L. J., Bernstein, M. P., & Sandford, S. A. (1997). Photochemical evolution of interstellar/precometary organic material. In Astronomical and Biochemical Origins and the Search for Life in the Universe, C.B. Cosmovici, S. Bowyer, & D. Werthimer (eds.), Proc. 5th International Conf. on Bioastronomy, IAU Coll. #161.
Many of these can be downloaded from our Publications page.
|