|
Amino acids are important biological molecules that serve as the basic molecular building blocks of proteins (including enzymes) used by all living things on Earth. All amino acids have a similar basic structure like that in Figure 1, with different amino acids differing only in the specific structure that lies in the chemical group or side carbon chain labeled “R”.
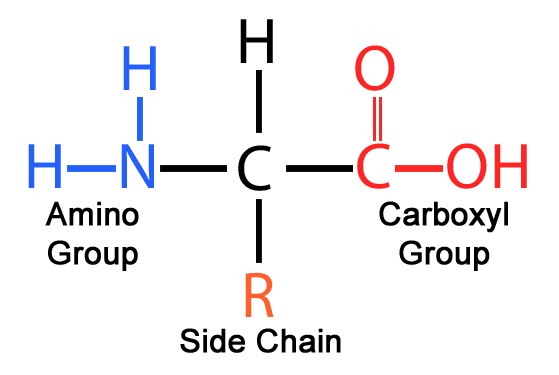
Figure 1: The basic structure of all amino acids.
As two examples, here are schematics of the simple amino acids glycine (R = H, a hydrogen atom) and alanine (R = CH3, a methyl group, Figure 2).
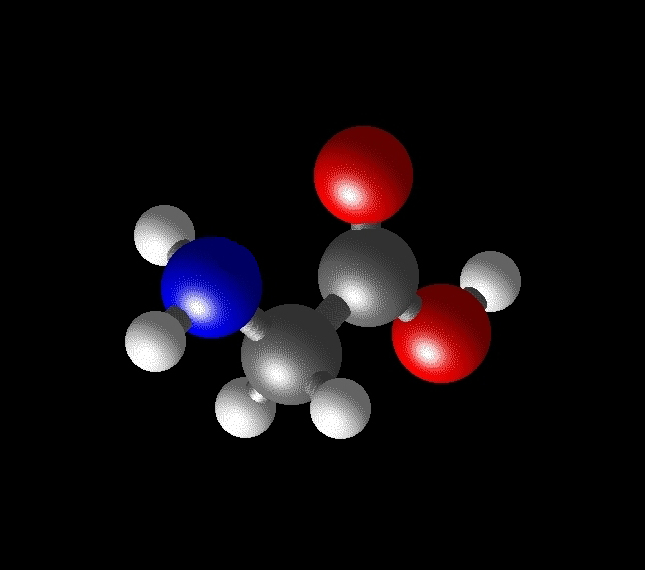 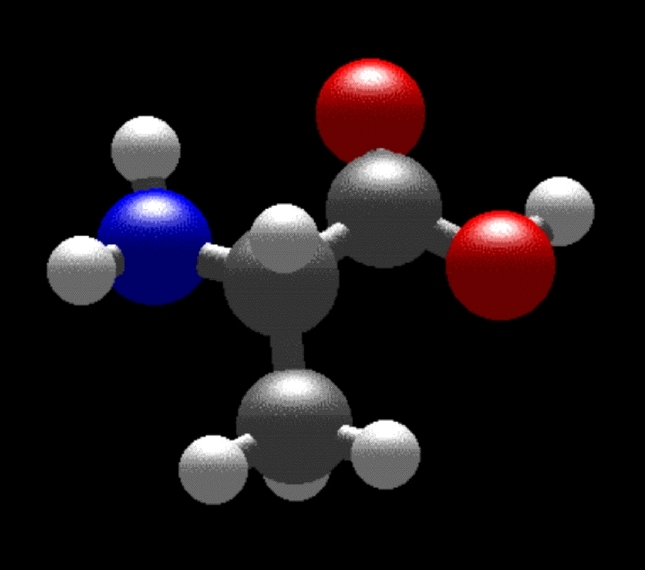
Figure 2: The structures of the simple amino acids glycine (left) and alanine (right).
The chemical group or side chain at position “R” can be almost anything, but life on Earth uses slightly over 20 different amino acids. Other amino acids are possible, however. For example, over 70 different amino acids have been identified in meteorites.
One of the peculiar properties of amino acids is that they are “chiral” or “handed” molecules (Figure 3). Because of the arrangement of chemical groups around the ‘central’ carbon atom of an amino acid, it is possible to make two versions (called enantiomers) of an amino acid that have identical chemical formulas, but that are geometrically different, each being the mirror image of the other. The situation is somewhat similar to your two hands, which have the same ‘formula’ (Palm1Fingers4Thumb1), but no amount of rotating of either hand can get the two to perfectly superpose. Keeping the analogy with your hands, the two enantiomers of an amino acid are often referred to as being ‘right-’ and ‘left-handed’ enantiomers of the same molecule.

Figure 3: Amino acids come in right and left handed versions.
Living creatures on Earth use only ‘left-handed’ amino acids. Interestingly, when amino acids are produced abiotically, i.e., via chemistry that does not involve living systems, the resulting amino acids are found to contain equal amounts of right- and left-handed enantiomers. This even mixture of both types is referred to as being ‘racemic.’ It is not currently understood why terrestrial biology uses only ‘left-handed’ amino acids, but there is a lot of speculation on how this may have come to be.
The Formation of Amino Acids in Interstellar Ices
Our work has shown that when mixed molecular ices of the sorts we see in space are irradiated by UV photons, amino acids are one of the types of products that are produced in measurable amounts. Figure 4 shows high-performance liquid chromatography (HPLC) data from a residue made from the UV photolysis of an ice containing H2O, CH3OH, CO, and NH3, indicating the identification of the amino acids serine, glycine, and alanine. Note the double peaks for serine and alanine, showing that both right- and left-handed enantiomers of these amino acids are made (glycine, where R = H, is not chiral). Other experiments show residues containing up 16 amino acids, both used and not used in modern biology to make proteins.
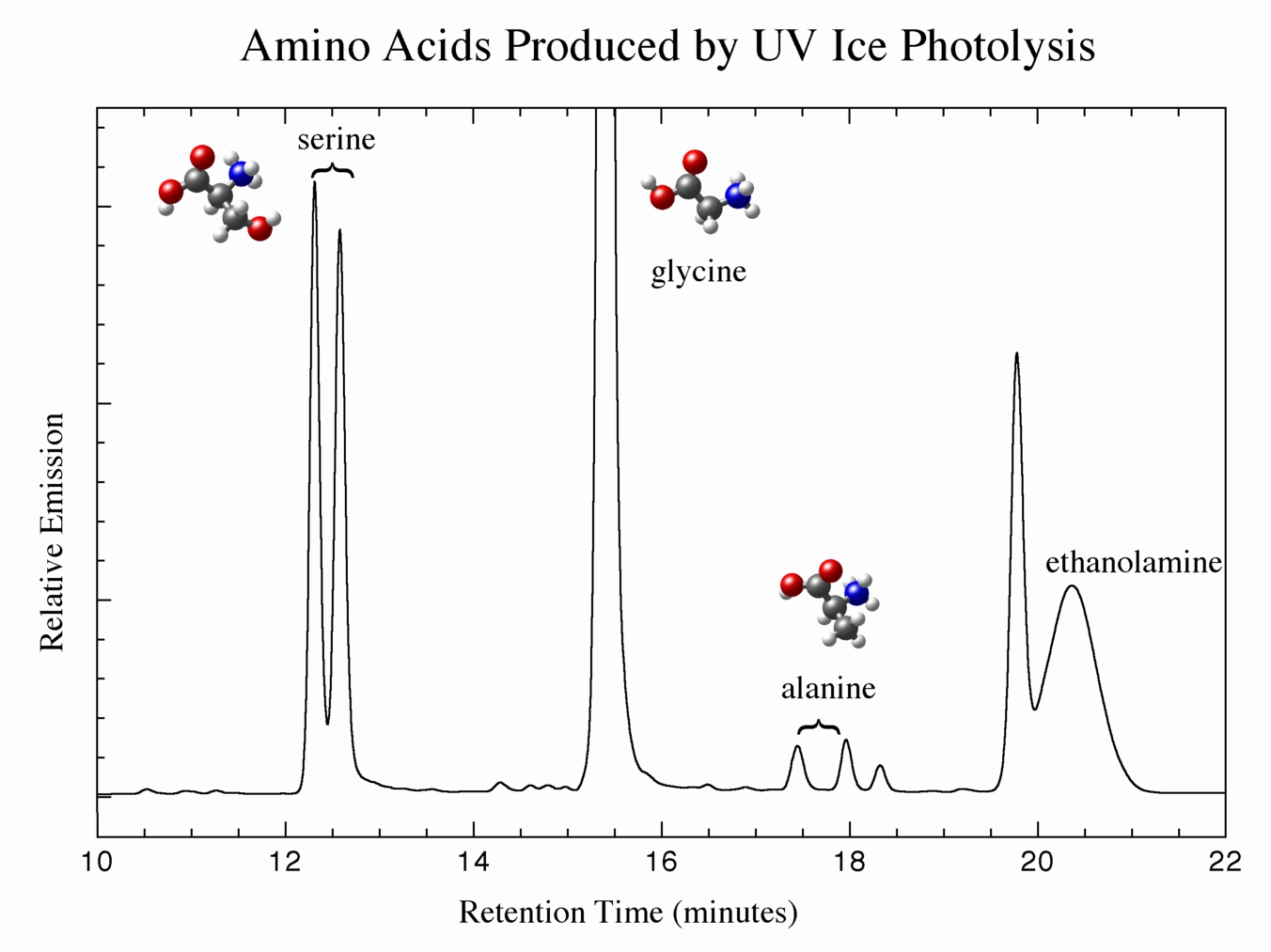
Figure 4: UV photolysis of ices of composition H2O:CH3OH:CO:NH3 yield amino acids as products.
Extensive sets of experiments have shown that the production of amino acids during ice photolysis is a relatively robust process. Most ices that contain C, H, O, and N within their original molecular components will yield several amino acids when irradiated by ionizing radiation such as UV photons or energetic protons. More details about our work on amino acids can be found in the references listed at the end of this page.
What is the Astrobiological Significance of the Formation of Amino Acids in Space?
This work shows that chemistry that occurs in space can lead to the production of amino acids, which are among materials that are routinely delivered to telluric planets including Earth, and that the presence of these materials could have played a key role in the origin of life on our planet. Since the process of ice photolysis is thought to occur wherever new stars and planets form, this implies amino acids may well be introduced to the surfaces of virtually all newly formed planets.
Amino acids are known to be present in primitive meteorites and many of these amino acids show the presence of excess deuterium (heavy hydrogen). Excess deuterium is an indicator of the presence of extraterrestrial materials, clearly demonstrating that extraterrestrial organic amino acids exist, survive planetary accretion, and are delivered to the Earth’s surface. Thus, the abiotic production of amino acids in space, along with sugar derivatives, compounds related to polycyclic aromatic hydrocarbons (PAHs), amphiphiles, hexamethylenetetramine (HMT), and nucleobases, may play a key role in the seeding of the surfaces of new planets with many of the building blocks needed to start life throughout the universe. It is interesting to note that, while meteorites are known to deliver over 70 varieties of amino acids to the Earth, modern life on the Earth uses only 20 of these. Why living systems use the particular amino acids they do is not currently understood.
For more detailed information and reviews on our laboratory work associated with amino acids, see:
Elsila, J. E., Dworkin, J. D., Bernstein, M. P., & Sandford, S. A. (2007). Mechanisms of Amino Acid Formation in Interstellar Ice Analogs. Astrophys. J. 660, 911–918.
Bernstein, M. P., Bauschlicher, C.W. Jr., & Sandford, S. A. (2004). The Infrared Spectrum of Matrix Isolated Aminoacetonitrile, a Precursor to the Amino Acid Glycine. Adv. Space Res. 33, 40–43.
Bernstein, M. P., Ashbourn, S., Sandford, S. A., & Allamandola, L. J. (2004). The Lifetimes of Nitriles (-C≡N) and Acids (-COOH) During Ultraviolet Photolysis and Their Survival in Space. Astrophys. J. 601, 365–370.
Bernstein, M. P., Dworkin, J. P., Sandford, S. A., Cooper, G. W., & Allamandola, L. J. (2002). The Formation of Racemic Amino Acids by Ultraviolet Photolysis of Interstellar Ice Analogs. Nature 416, 401–403.
Many of these can be downloaded from our Publications page.
|