|
Amphiphiles are long molecules that have a polar, hydrophilic (water-loving) ‘head’ group and a hydrophobic (water-fearing) ‘tail’. Molecules of this sort are common in detergents: in the presence of dirt they will orient themselves to surround the particles with their tails on the particle (away from the water) and their heads facing outward (into the water). This effectively couples the particle to the surrounding water and makes it easier to wash away.
In the absence of particles, amphiphiles must find another way to ‘hide’ their hydrophobic tails from surrounding water molecules. If they are long enough and are at the correct concentration and pH, these molecules can spontaneously self-assemble into more complex structures (see Figure 1). One way they can get their tails away from water is to form a film at the surface with their tails sticking out of the water. Another way to hide their tails is to form a spherical shell with all their tails pointing inward. This sort of structure is called a micelle. Larger structures can be made when the individual amphiphiles line up in parallel sheets with their tails together; this sort of structure is called a bilayer.
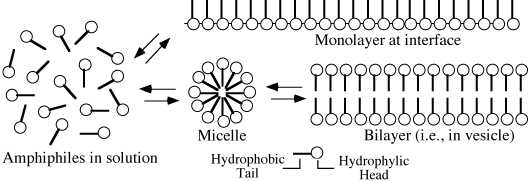
Figure 1: Different ways for amphiphilic molecules to
minimize their energy when put in water by turning their hydrophilic ‘head’
towards water and turning their hydrophobic ‘tails’ away from water.
If a bilayer sheet wraps around on itself, it can form a more spherical bilayer called a vesicle, a hollow blob surrounded by a bilayer membrane (Figure 2). This is the basic structure that makes up the walls of cells in living things.
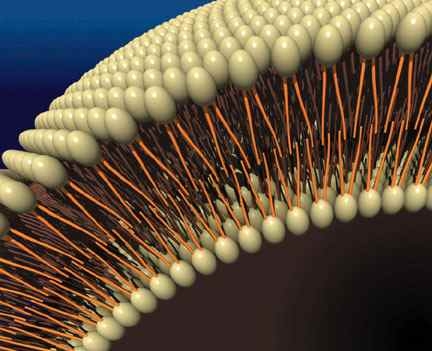
Figure 2: The structure of a bilayer membrane.
What do Amphiphiles have to do with the UV Photolysis of Interstellar and Cometary Ices?
In our laboratory, we simulate the thermal and photochemical processes that occur in mixed molecular ices in physical environments like interstellar dense molecular clouds, protostellar disks, comets, and outer regions of our Solar System (for more details about these simulations, click here). Energetic processing of these ices has been shown to convert simple, abundant molecules (like H2O, CH3OH, NH3, CO, CO2, CH4, etc.) into more complex organic materials. The resulting populations of organics are very complex and include a number of classes of compounds that are of astrobiological interest, including amino acids, PAH-related compounds, hexamethylenetetramine, nucleobases, and the amphiphiles discussed here.
The presence of newly created amphiphiles in the organic residues that result from UV irradiation of astrophysical ice analogs can be demonstrated by putting the residues into liquid water. Indeed, when these residues are placed in water, one or more of the compounds present spontaneously form membranes that produce “vesicles” of the sort shown in Figure 3 . For more details, see Dworkin et al. (2001). The left-hand image shows what the vesicles look like in visible light under a microscope. Interestingly, the membranes in these structures fluoresce, that is, they absorb UV radiation and re-emit the energy at visible wavelengths. The right hand image shows the same field of view as the left hand image, the difference being that in the right-hand image, the sample is being illuminated by UV light and the vesicles are “glowing.”
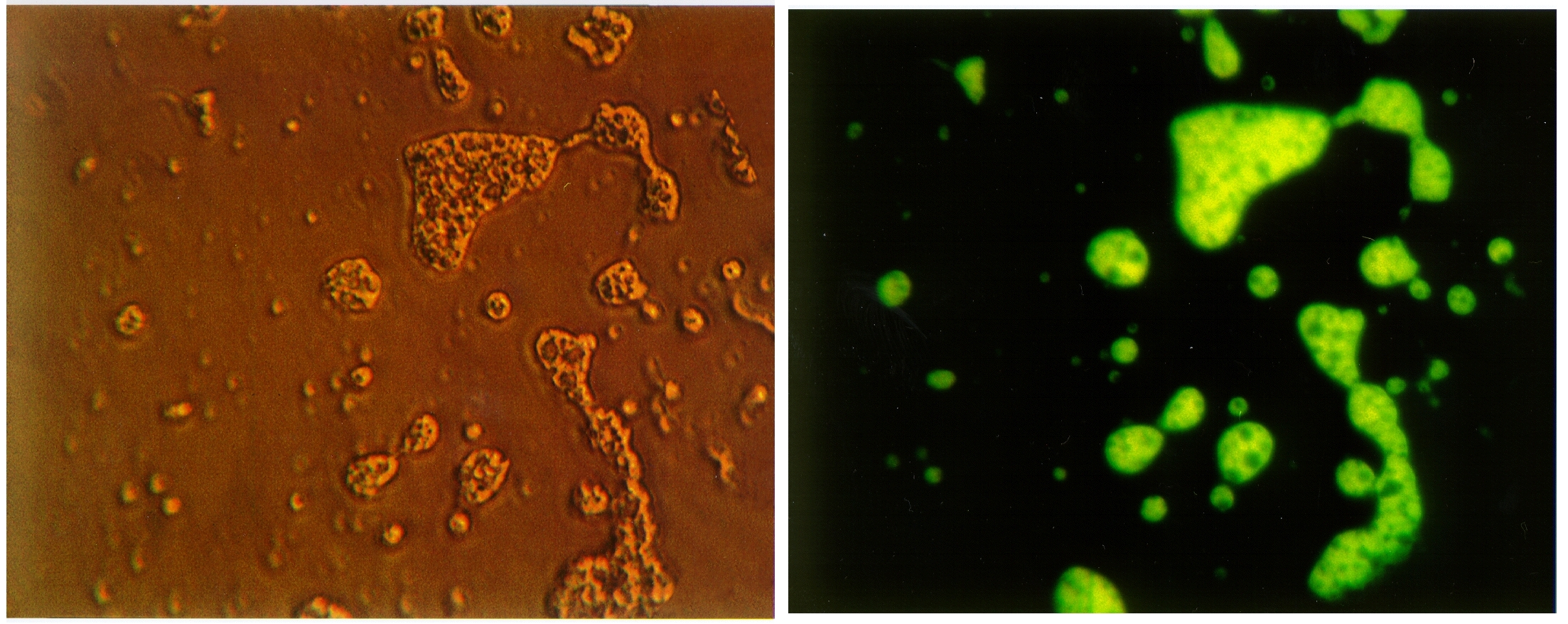
Figure 3: Vesicles forming spontaneously when organic residues are placed
in water and the amphiphiles present self-assemble into these structures.
What is the Astrobiological Significance of Amphiphiles?
This work shows that the chemistry that occurs in the depths of space can lead to the production of a number of materials that play key roles in biochemistry on Earth. These processes are thought to be occurring throughout the galaxy, implying that the vastness of space is filled with compounds that, if introduced to a hospitable environment like the surface of a suitable planet, could play key roles in the origin of life.
Amphiphiles are present in the Murchison meteorite (Deamer, 1985, Nature 317, 792–794) and are produced in our interstellar ice simulation experiments (Dworkin et al. 2001). Such self-assembling molecules probably played a key role in the emergence of life because of the unique advantages they can provide (Deamer et al. 2002). For example, enclosed vesicular structures are capable of concentrating molecules, overcoming dilution, and facilitating interactions that develop or maintain macromolecules. Moreover, since the aromatic components of the prebiotic organic inventory include non-polar pigments, they have the potential to capture light energy and screen the interior from damaging UV light when partitioned into the hydrophobic phase of a bilayer. This emergent set of biophysical properties can arise from amphiphilic molecules that have the ability to self-assemble into more complex vesicular structures.
Thus, the abiotic production of amphiphiles in space, along with amino acids, PAH-related compounds, hexamethylenetetramine, and nucleobases, may play a critical role in the seeding of the surfaces of new planets with many of the building blocks needed to start life throughout the universe.
For more detailed information and reviews on our laboratory work associated with amphiphiles, see:
Deamer, D. W., Dworkin, J. P., Sandford, S. A., Bernstein, M. P., & Allamandola, L. J. (2002). The First Cell Membranes. Astrobiology 2, 371–381.
Dworkin, J. P., Deamer, D. W., Sandford, S. A., & Allamandola, L. J. (2001). Self-Assembling Amphiphilic Molecules: Synthesis in Simulated Interstellar/Precometary Ices. Proc. Natl. Acad. Sci. 98, 815–819.
Many of these can be downloaded from our Publications page.
|