What are Polycyclic Aromatic Hydrocarbons (PAHs)?
Polycyclic aromatic hydrocarbons (PAHs) represent one of the most abundant forms of carbon in the universe. They are seen in a wide variety of environments in space, and many varieties of these molecules are seen in meteorites as well as asteroidal and cometary dust.
Canonical PAHs consist of fused groups of six-sided carbon rings laid out in a planar structure. Figure 1 shows some examples of PAHs containing different numbers and arrangements of rings, although the number of possible arrangements or rings is enormous. In the structures in the image below, each vertex represents a carbon atom. A single hydrogen atom is attached to each external vertex.
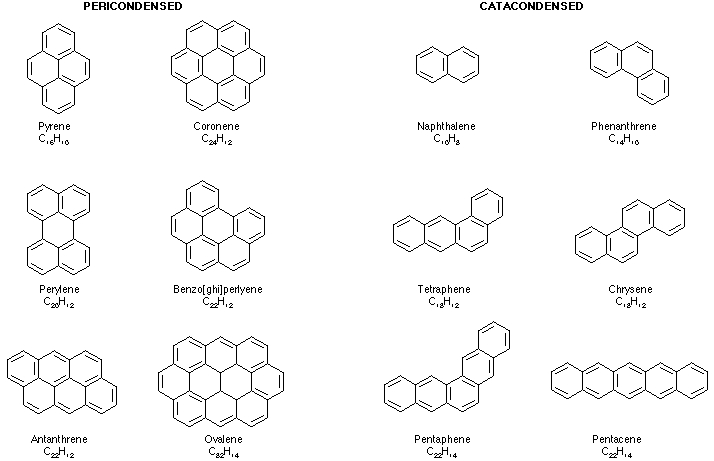
Figure 1: Chemical structures of several PAH compounds
Which contain 2 (naphthalene) to 10 (ovalene) aromatic rings.
Variants on these structures can also exist. For example, it is possible for some six-membered rings to be replaced with five- or seven-membered rings. This results in a curvature of the plane of the molecule. It is also possible to replace some of the carbon atoms in the rings with other elements, for example, heteroatoms such as nitrogen and oxygen. Finally, it is also possible to ‘decorate’ PAHs by replacing one or more of their peripheral hydrogen atoms with some other chemical group, for example, a methyl (–CH3) group or an alcohol (–OH) group.
PAH Chemistry in Space
PAHs may form in a number of different environments in space, but it is thought that most of them form in the outflows of carbon-rich stars. Once formed, however, PAHs are available to participate in further chemistry that can modify them in a number of interesting ways.
Since most PAHs are relatively non-volatile compounds, they are probably frozen out into the ices that coat cold dust grains in dense interstellar clouds. The ices in dense interstellar clouds are dominated by the molecule H2O, but also contain many other simple species like methanol (CH3OH), carbon monoxide (CO), carbon dioxide (CO2), ammonia (NH3), methane (CH4), formaldehyde (H2CO), as well as other simple species.
Other work in our laboratory shows that the exposure of these ices to high-energy radiation in the form of UV photons or charged particles can result in the production of a host of new molecular compounds, some of which are of astrobiological interest. What happens when PAHs are present in these ices?
Over the years we have studied the photochemistry that occurs when PAHs are placed in ices of different compositions and exposed to hard radiation. We have discovered that PAHs exposed to radiation in H2O-rich ices are easily ionized and that this can lead to a host of chemical substitutions involving their peripheral hydrogen atoms. The nature of the substitution depends on the composition of the surrounding ice. The table below shows a number of substitutions in which the site of a peripheral hydrogen atom has been altered by the addition of an extra hydrogen atom (Hn-PAH) or replaced by another chemical group like =O, –OH, –NH2, –OCH3, –COOH, –CH3, and –CN/–NC (Figure 2).
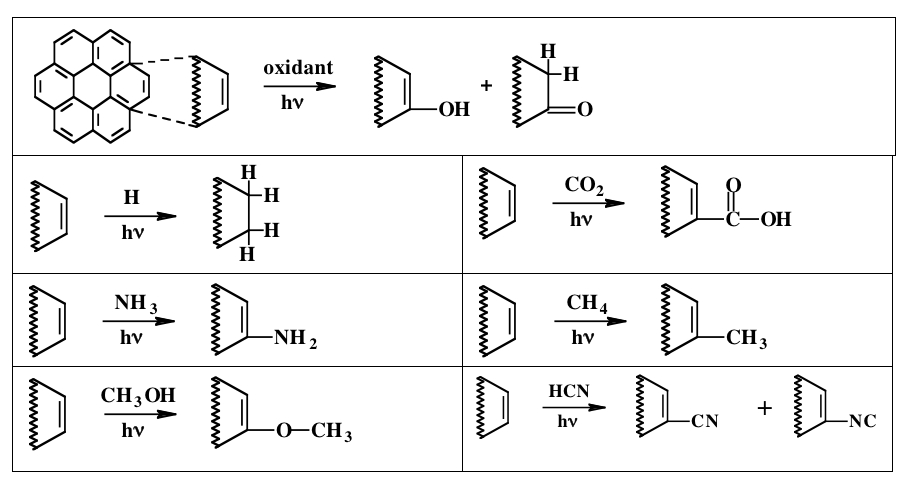
Figure 2: The UV irradiation of PAHs in astrophysically relevant ice mixtures results in the addition of H atoms or different chemical groups to their peripheral carbon atoms.
In the sections below we discuss several of these types of substitutions and their implications for astrochemistry and astrobiology.
Quinones and Other Oxidized PAHs in Space
Interstellar ices are generally dominated by the molecule H2O. When PAHs are photolyzed in H2O ices, the dominant reactions that occur are H and O-atom addition reactions. Oxygen addition reactions can lead to the formation of aromatic ethers, alcohols, and ketones (see Figure 3). These kinds of compounds are seen in carbon-rich meteorites and some of them are of astrobiological interest.
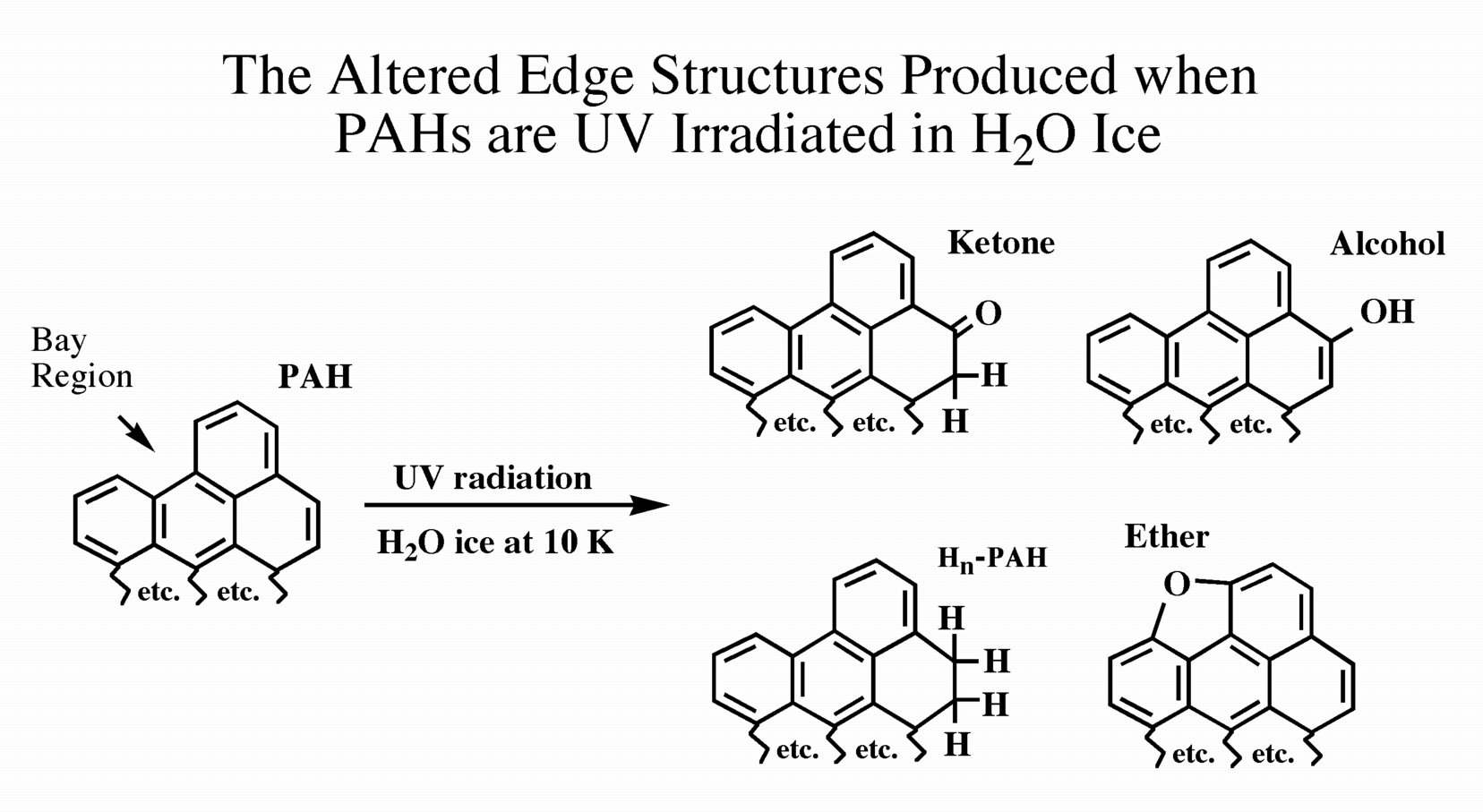
Figure 3: The UV irradiation of PAHs in H2O ice results in the
formation of Hn-PAHs and PAHs with ketone, alcohol, and ether groups.
Of particular interest are oxidized PAHs that have C=O groups on their edges, i.e., aromatic ketones. The aromatic ketones contain a class of compounds known as quinones, which play a variety of key biochemical roles in living organisms on Earth.
As an example, consider the two-ring PAH naphthalene. When this molecule is frozen into an ice containing H2O and exposed to UV radiation, oxygen atoms can be added to the edges of the naphthalene, forming a number of new compounds. Some of the most abundant products are the aromatic alcohols 1-naphthol and 2-naphthol, and the quinone 1,4-naphthoquinone (see Figure 4).
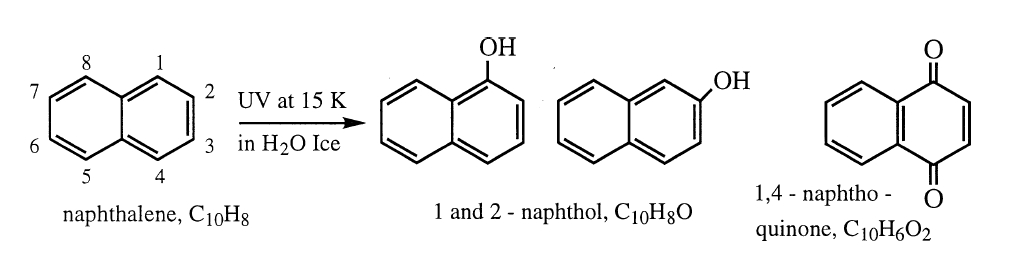
Figure 4: The UV irradiation of naphthalene (C10H8) in H2O-rich ices results
in the addition of alcohol (–OH) groups or ketones (=O) and the formation of naphthols and naphthoquinones, some of which are important molecules used in modern biology.
The production of naphthoquinones is of particular interest since this class of compounds includes important biomolecules such as the vitamin K family that plays a key role in electron transport in living systems. It has also been proposed that naphthoquinones may have been among the first chromophores employed by the first organisms to protect themselves from UV radiation before the atmosphere contained a protective ozone layer. Quinones produced in space from the irradiation of PAHs may therefore have played a role in seeding the surface of Earth with important compounds that may have played a role in the emergence and evolution of life on Earth and maybe elsewhere.
Hydrogenated Polycyclic Aromatic Hydrocarbons (Hn-PAHs) in the Interstellar Medium
Hn-PAHs are PAHs that carry excess H atoms on one or more of their peripheral carbon atoms. Molecules of this type may be produced in the same carbon star outflows that are thought to produce normal PAHs. As noted above, these molecules can also be formed when regular PAHs are irradiated in ices that contain other molecular species that bear hydrogen atoms.
The carbon bonding in normal PAHs with six-membered carbon rings is all sp2 and the resulting molecules are all planar. However, the addition of an extra hydrogen atom to a carbon atom in the molecule converts that atom to sp3 tetrahedral bonding, which results in distortions of the molecule from its original planar shape. The resulting molecules produce IR spectra that show elements of their mixed aromatic and aliphatic bonding (Sandford et al. 2013).
These types of molecules may explain some of the more enigmatic spectral features seen in some IR emission objects. For example, the IR emission in the C–H stretching region from the Orion Bar (an HII region) is shown below compared with our lab spectrum of hexahydropyrene (H6-pyrene) isolated in an argon matrix (Figure 5) .
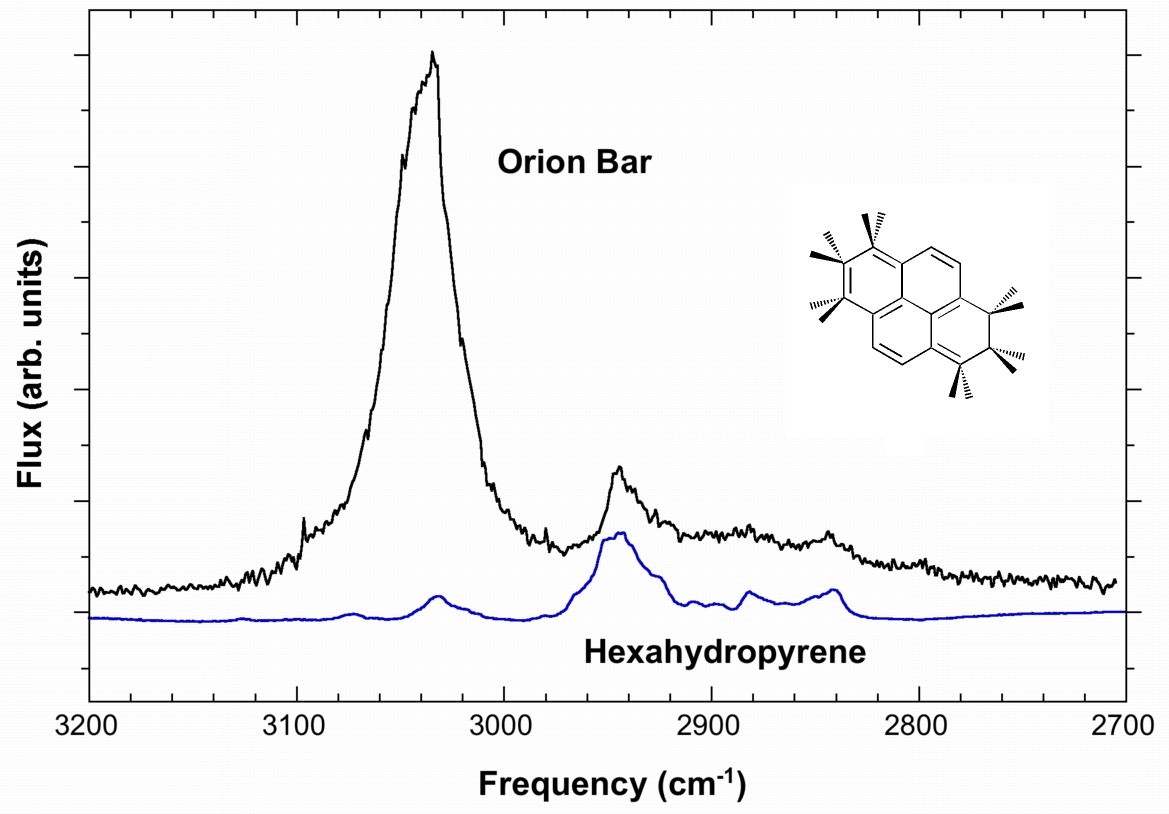
Figure 5: Comparison between the IR emission spectra of the Orion Bar and of hexahydropyrene (H6-pyrene), a hydrogenated PAH (Hn-PAH), isolated in an argon matrix.
The 3040-cm-1 feature in the spectrum in Figure 5 is thought to be due to the normal aromatic C–H stretch of PAHs, but the precise identification of the weak features longward of the 3040-cm-1 emission feature have remained somewhat enigmatic. In most objects, these features, falling near 2940, 2890, 2850, and 2810 cm-1 (3.40, 3.46, 3.51, and 3.56 μm), are weak compared to the 3040 cm-1 (3.29 μm) band and show a tendency to decrease in strength with decreasing frequency. However, in the spectra of a small number of early type objects spanning the evolutionary bridge between carbon-rich giants and planetary nebulae, the 2940 and 2850 cm-1 bands (3.40 and 3.51 μm) are actually stronger than the 3040-cm-1 feature. As the spectrum of hexahydropyrene shows, PAHs containing excess H atoms (Hn-PAHs) may be able to explain this (Materese et al. 2017).
The Formation of Aromatic Heterocycles
The irradiation of PAHs in ices containing H2O, CH3OH, NH3, and other simple molecules can also produce new aromatic compounds in which one of the C atoms in the original PAH structure is replaced by a heteroatom like N or O, see Figure 6 (Materese et al. 2014).
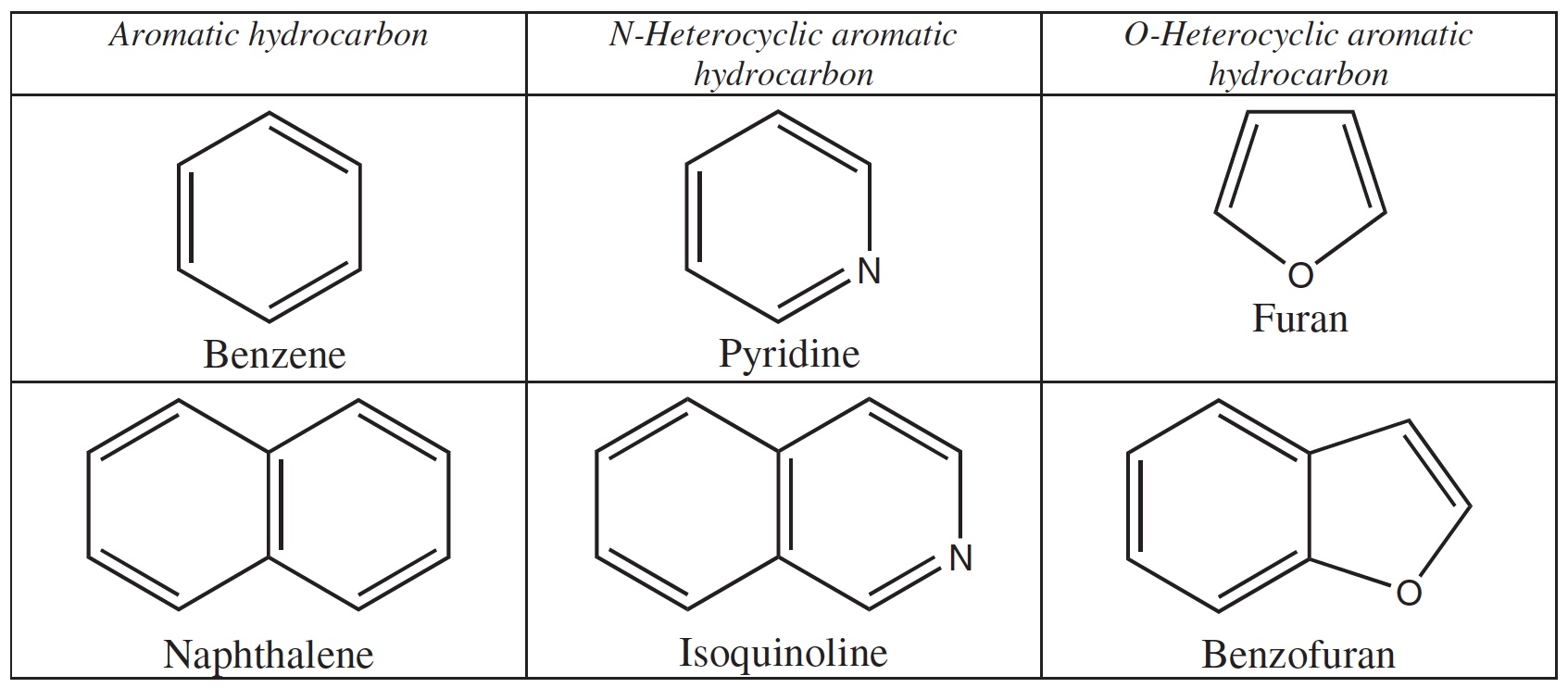
Figure 6: Examples of aromatic heterocycles in
which carbon atoms are replaced by N or O atoms.
The mechanism by which this substitution occurs during ice irradiation is not yet fully understood, but it may involve the substitution of a peripheral H atom on the original PAH with an N- or O-containing side group (see Figure 2), followed by the breaking of the adjacent ring structure and rotation of the side group into the ring.
The production of aromatic heterocycles from PAHs has astrobiological implications since N-containing heterocycles play many key roles in modern biochemistry. For example, the five nucleobases contained in RNA and DNA are all based on the structures of the N-heterocyclic molecules pyrimidine and purine (see Nucleobases).
For more detailed information and reviews on our laboratory work on quinones, Hn-PAHs, and other products made during the photoprocessing of aromatic molecules in ices, see:
Materese, C. K., Bregman, J. D., & Sandford, S. A. (2017). The Detection of 6.9 μm Emission Features in the Infrared Spectra of IRAS 04296+3429, IRAS 05341+0852, IRAS 22272+5435: Evidence for the Presence of Hn-PAHs in Post AGB Stars. Astrophys. J. 850, 165 (5 pp).
Materese, C. K., Nuevo, M., & Sandford, S. A. (2014). N- and O-heterocycles Produced from the Irradiation of Benzene and Naphthalene in H2O/NH3-Containing Ices. Astrophys. J. 800, 116 (8 pp).
Sandford, S. A., Bernstein, M. P., & Materese, C. K. (2013). The Infrared Spectra of Polycyclic Aromatic Hydrocarbons with Excess Peripheral H Atoms (Hn-PAHs) and their Relationship to the 3.4 and 6.9 μm PAH Emission Features. Astrophys. J. Suppl. Ser. 205, 8 (30 pp).
Ashbourn, S. F. M., Elsila, J., E. Dworkin, J. P., Bernstein, M. P., Sandford, S. A., & Allamandola, L. J. (2007). Ultraviolet Photolysis of Anthracene in H2O Interstellar Ice Analogs: Potential Connection to Meteoritic Organics. Meteorit. Planet. Sci. 42, 2035–2041.
Bernstein, M. P., Sandford, S. A., Mattioda, A. L., & Allamandola, L. J. (2007). Near- and Mid-Infrared Laboratory Spectra of PAH Cations in Solid H2O. Astrophys. J. 664, 1264–1272.
Bernstein, M. P., Moore, M. H., Elsila, J. E., Sandford, S. A., Allamandola, L. J., & Zare, R. N. (2003). Side group Addition to the PAH Coronene by Proton Irradiation in Cosmic Ice Analogs. Astrophys. J. 582, L25–L29.
Bernstein, M. P., Elsila, J. E., Dworkin, J. P., Sandford, S. A., Allamandola, L. J., & Zare, R. N. (2002). Side group Addition to the PAH Coronene by UV Photolysis in Cosmic Ice Analogs. Astrophys. J. 576, 1115–1120.
Bernstein, M. P., Dworkin, J., Sandford, S. A., & Allamandola, L. J. (2001). Ultraviolet Irradiation of Naphthalene in H2O Ice: Implications for Meteorites and Biogenesis. Meteorit. Planet. Sci. 36, 351–358.
Sandford, S. A., Bernstein, M. P., Allamandola, L. J., Gillette, J. S., & Zare, R. N. (2000). Deuterium Enrichment of PAHs by Photochemically Induced Exchange with Deuterium-Rich Cosmic Ices. Astrophys. J. 538, 691–697.
Bernstein, M. P., Sandford, S. A., Allamandola, L. J., Gillette, J. S., Clemett, S. J., & Zare, R. N. (1999). UV Irradiation of Polycyclic Aromatic Hydrocarbons in Ices: Production of Alcohols, Quinones, and Ethers. Science 283, 1135–1138.
Sloan, G. C., Bregman, J. D., Geballe, T. R., Allamandola, L. J., & Woodward, C. E. (1997). Variations in the 3 Micron Spectrum Across the Orion Bar: Polycyclic Aromatic Hydrocarbons and Related Molecules. Astrophys. J. 474, 735–740.
Bernstein, M. P., Sandford, S. A., & Allamandola, L. J. (1996). Hydrogenated Polycyclic Aromatic Hydrocarbons (Hn-PAHs) and the 2940 and 2850 Wavenumber (3.40 and 3.51 Micron) Infrared Emission Features. Astrophys. J. 472, L127–L130.
Many of these can be downloaded from our Publications page.
|