|
Near infrared (NIR) reflection spectra of outer Solar System objects show that CO2 occurs on the solid surfaces of several bodies in the Solar System. In addition to the seasonal deposits of solid CO2 on Mars, carbon dioxide in the comae of some comets is presumed to evaporate from solid CO2 in their nuclei. Grundy (2003) found three bands of solid CO2 in the 2 micron region in the spectrum of the Uranian satellite Ariel. Carbon dioxide is also seen in the reflectance spectra of Jupiter's satellites Europa, Ganymede and Callisto (McCord et al. 1997, 1998; Hibbits et al. 2000, 2003) and Saturn's satellites Phoebe (Clark et al. 2005) and Iapetus (Buratti et al. 2005). In all of these cases, the CO2 stretching fundamental band usually at 4.27 micron is shifted slightly to shorter wavelength (4.26 micron) and is presumed to originate from CO2 that is complexed in some way with other surface materials. The occurrence of CO2 in the form of fluid or gaseous inclusions in minerals has been suggested by the authors of the papers cited above.
Solid H2O is ubiquitous in the outer Solar System (Roush, 2001), and it is inevitable that CO2 will come into intimate contact with H2O at various temperatures and in varied proportions. For example, reflectance spectra of icy Galilean and Saturnian satellites show strong NIR absorptions of CO2 and H2O. Although good NIR spectra of pure CO2 and CO2 in N2 have been published (Hansen 1997, Quirico & Schmitt 1997, Gerakines et al., 2005), to our knowledge no NIR spectra of CO2 in H2O are available. The interaction between CO2 and H2O (and CH3OH) on a molecular level has been shown to cause significant changes in the position and profile of CO2 peaks in the mid-IR (Sandford & Allamandola 1990; Dartois et al., 1999; Ehrenfreund et al., 1999; Palumbo and Baratta 2000), so it seems reasonable that the presence of CO2 could change NIR CO2 peaks as well. Indeed, in this paper we show that one such peak exists, the classically 'forbidden' 2ν3 overtone near 2.134 micron. This could potentially complicate the interpretation of IR of spectra of ices on outer Solar System bodies.
On this web page we display eight figures with NIR spectra of CO2-H2O and CH3OH-CO2 ice mixtures at temperatures from 15 to 150 K. We highlight the enhancement of the putative CO2 (2ν3) overtone near 2.134 micron (4685 cm-1) and its potential as an observational (spectral) indicator of whether solid CO2 is a pure material or intimately mixed with other molecules. As far as we know this is by far the most sensitive indicator CO2 intermolecular interactions. Even in cases where spectra of a surface show H2O and CO2 together, they could still be in small patches or in layers. Only a molecular approach would allow us to distinguish between these types of mixtures.
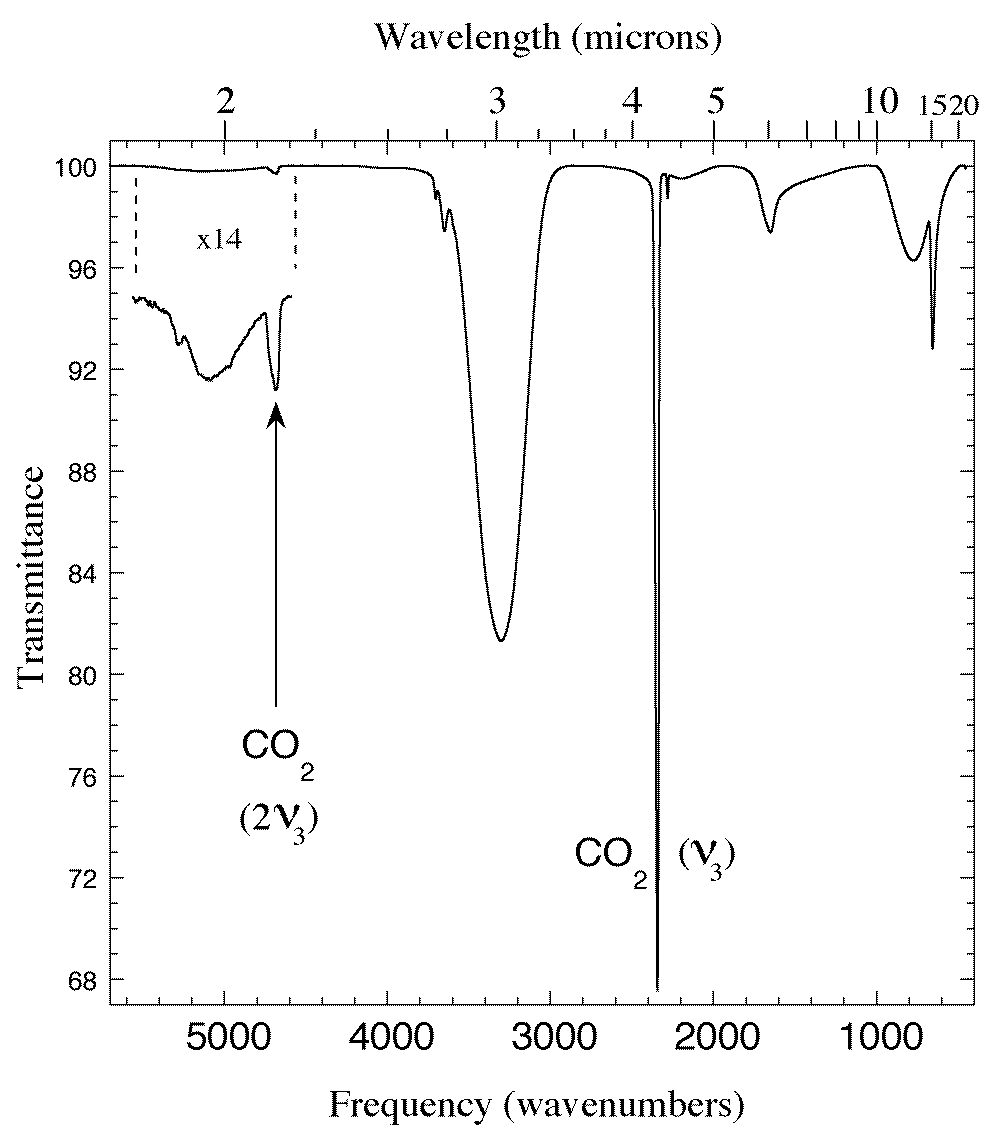
Figure 1. The 1.75-22 micron (5700-450 cm-1) IR spectrum of an H2O/CO2 = 5 ice mixture at 15 K. In addition to the broad absorptions of amorphous solid H2O and the sharper CO2 fundamentals one sees the 'forbidden' 2ν3 overtone of CO2 at 2.135 micron (4684 cm-1). This feature is prominent in spectra of mixtures but is not seen in spectra of pure CO2 (see Fig. 3).
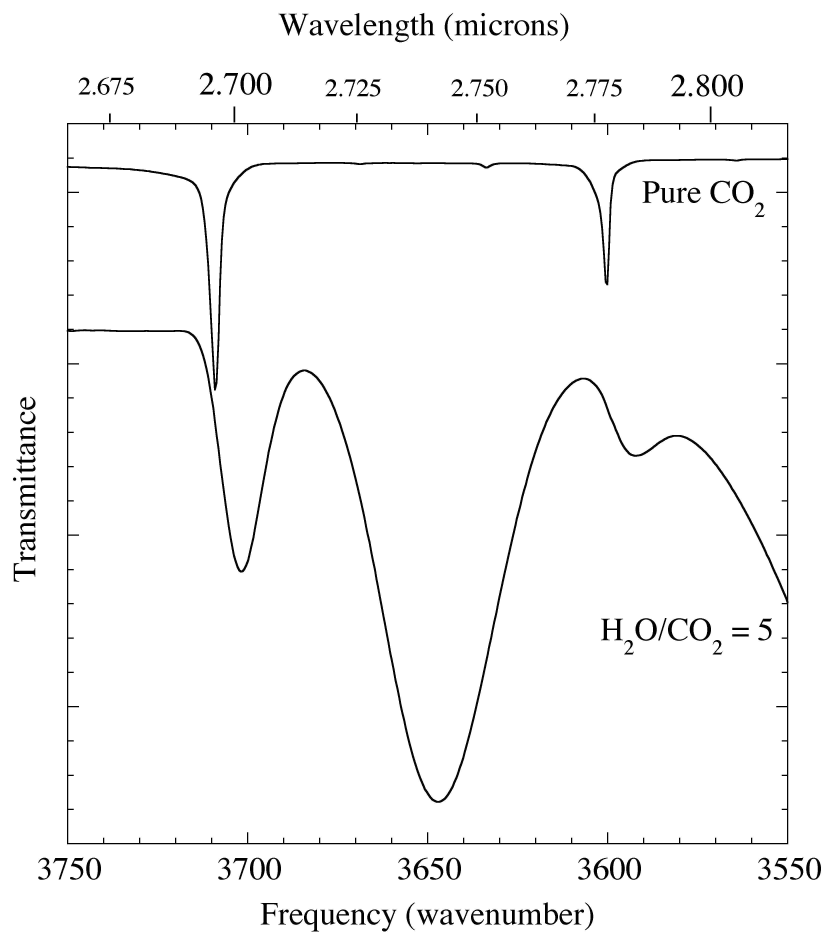
Figure 2. The 2.667-2.817 micron (3750-3550 cm-1) IR spectrum of pure CO2 (above) compared with that of an H2O/CO2 = 5 ice mixture at 15 K (below). The ~2.70 and 2.78 micron (3702 & 3592 cm-1) absorptions of CO2 in H2O are significantly broader and shifted to longer wavelength than those of pure CO2. The central broad feature near 2.74 micron (3650 cm-1) is an absorption of H2O, not CO2, so it does not appear in the upper spectrum. The lower spectrum drops off to the right because of the large 3 micron H2O band (see Fig. 1).
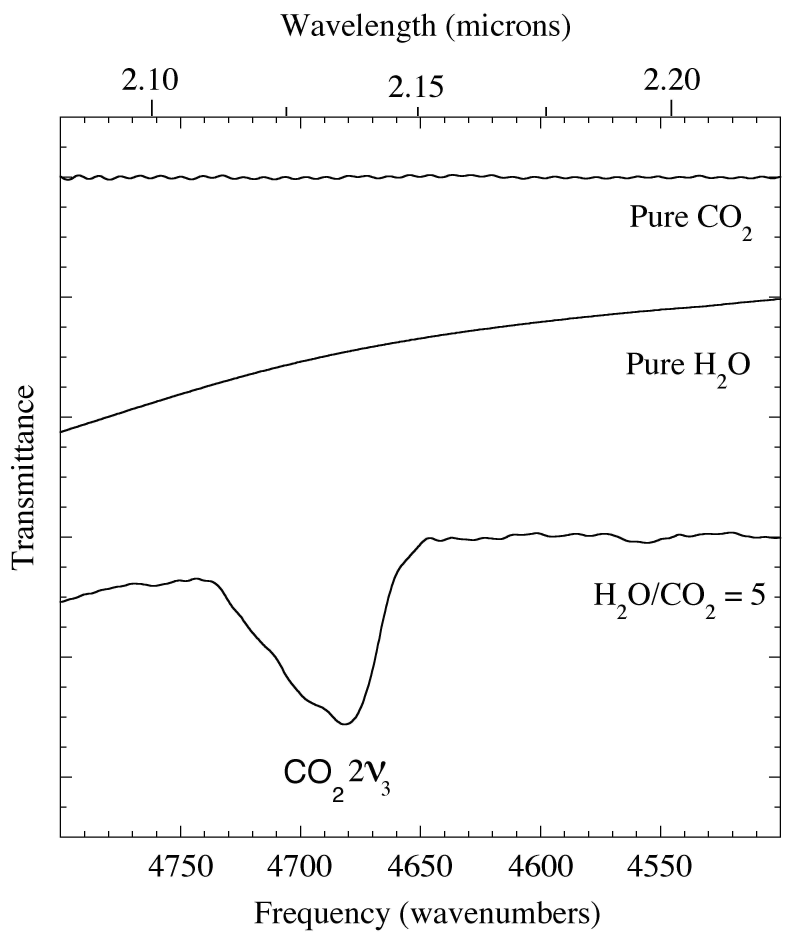
Figure 3. The 2.08-2.27 micron (4800-4400 cm-1) IR spectra of pure CO2 and H2O compared with that of an H2O/CO2=20 ice. The ν2v3 overtone of the asymmetric stretch of CO2 near 2.135 micron (4684 cm-1) is absent from the spectra of pure CO2 and pure H2O, but present in the spectra of the CO2 mixed with H2O. The position and profile of the this overtone is sensitive to ice composition and concentration, not unusual behavior for a classically 'forbidden' feature. The baselines of the spectra rich H2O in drop off to the left because of the 2 micron H2O band (see Fig. 1).
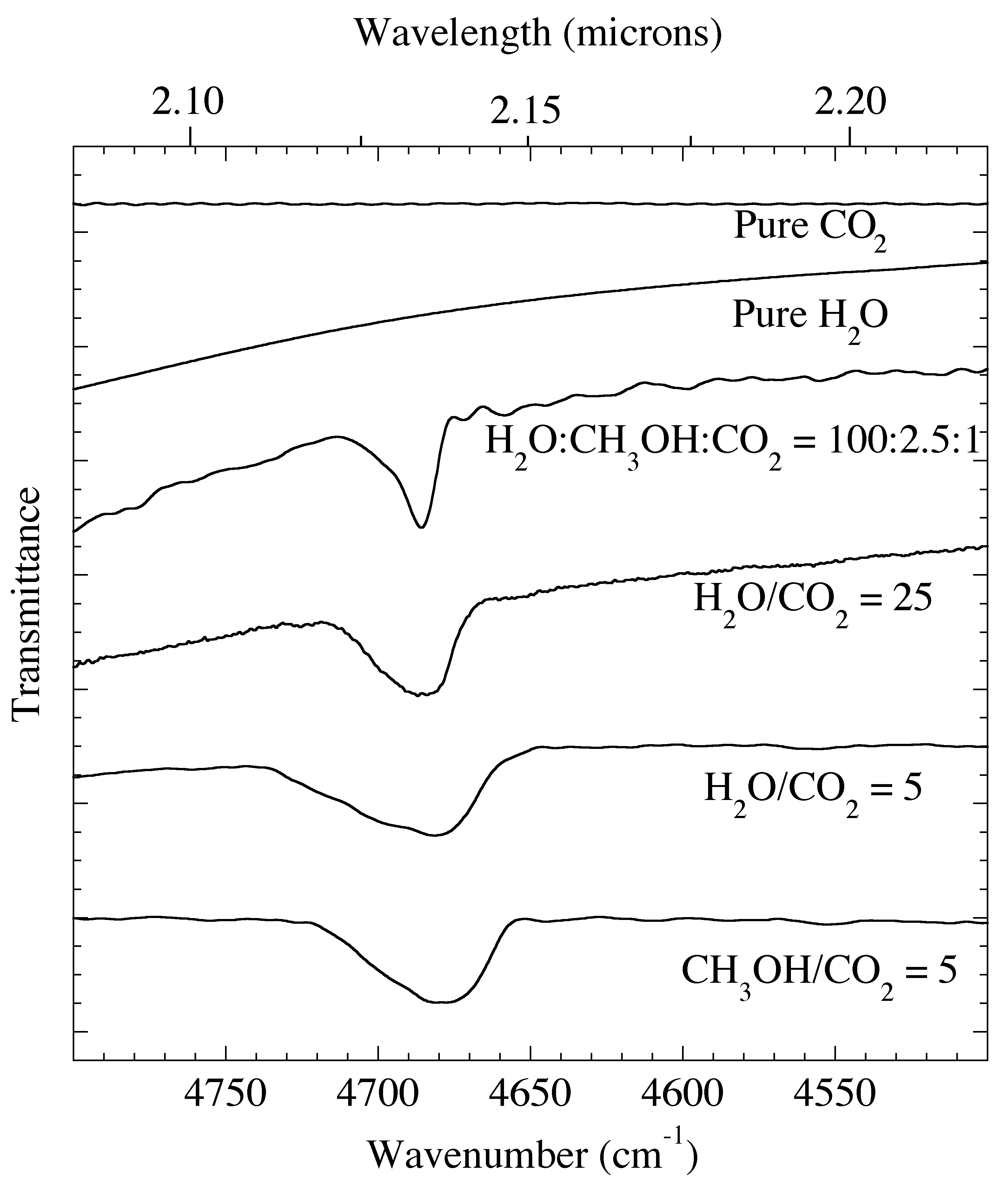
Figure 4. The 2.08-2.27 micron (4800-4400 cm-1) IR spectra of pure CO2 and H2O compared with various H2O, CH3OH, & CO2 containing ice mixtures at 15 K. The 2ν3 overtone of the asymmetric stretch of CO2 near 2.135 micron (4684 cm-1) is also present in the spectra of these mixed ices.
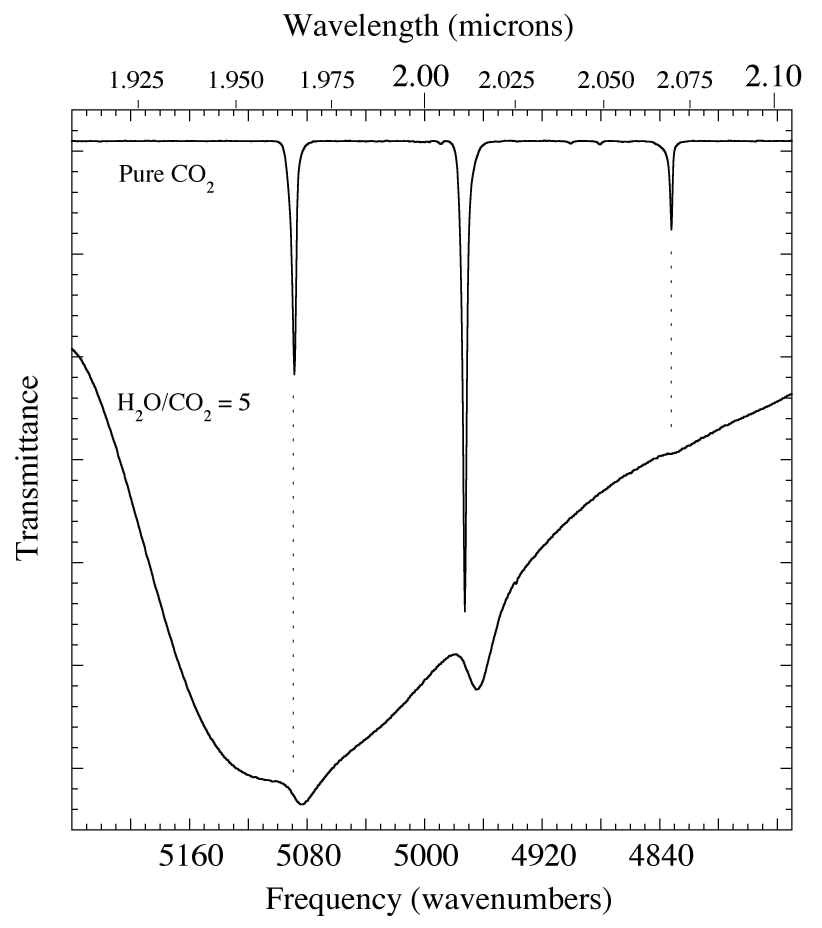
Figure 5. The 1.908-2.105 micron (5240-4750 cm-1) IR spectrum of pure CO2 (above) compared with that of an H2O/CO2 = 5 ice mixture (below) at 15 K. The large broad dip crossing the entire lower trace is caused by H2O. The smaller peaks at ~ 1.97, 2.01, and 2.07 micron (5083, 4963, and 4827 cm-1) are from CO2 in H2O. Compared to pure CO2 these absorptions from the mixture are significantly broader and shifted to longer wavelength.
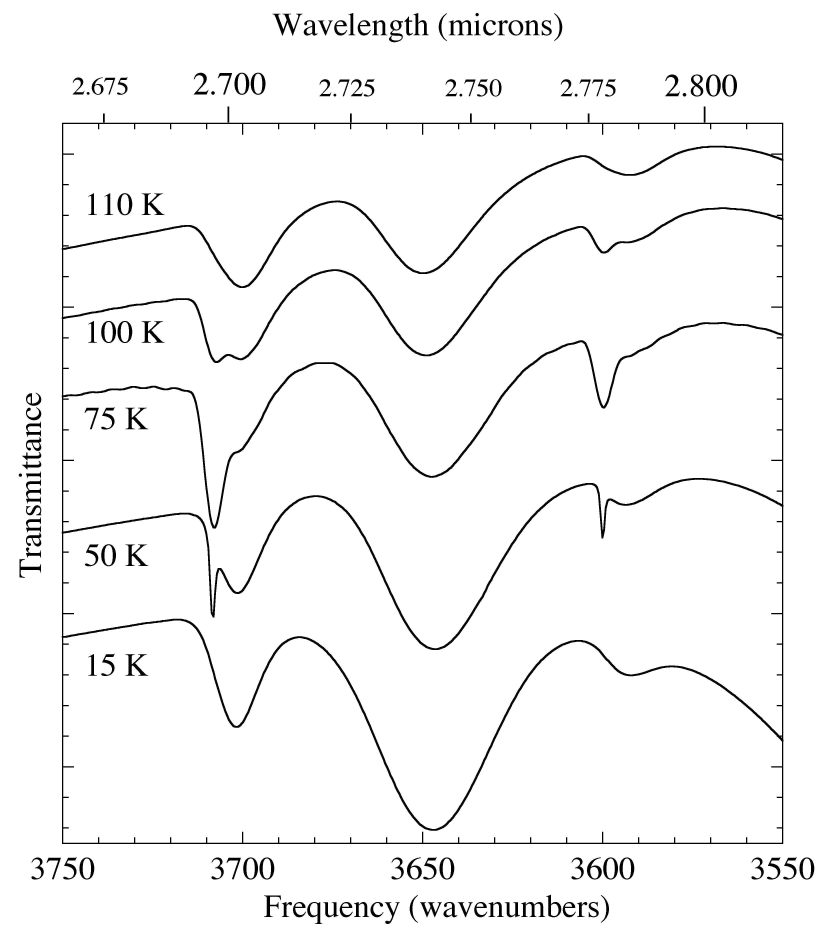
Figure 6. Temperature dependence of the 2.67-2.82 micron (3750-3550 cm-1) IR spectrum of an H2O/CO2 = 5 ice mixture. The broad features at ~2.70 and 2.79 micron (3700 and 3590 cm-1) of CO2 in H2O
are visible over the entire temperature range. The sharper peaks superimposed upon them that appear, broaden, and then diminish with
warming are similar to those produced by pure CO2.
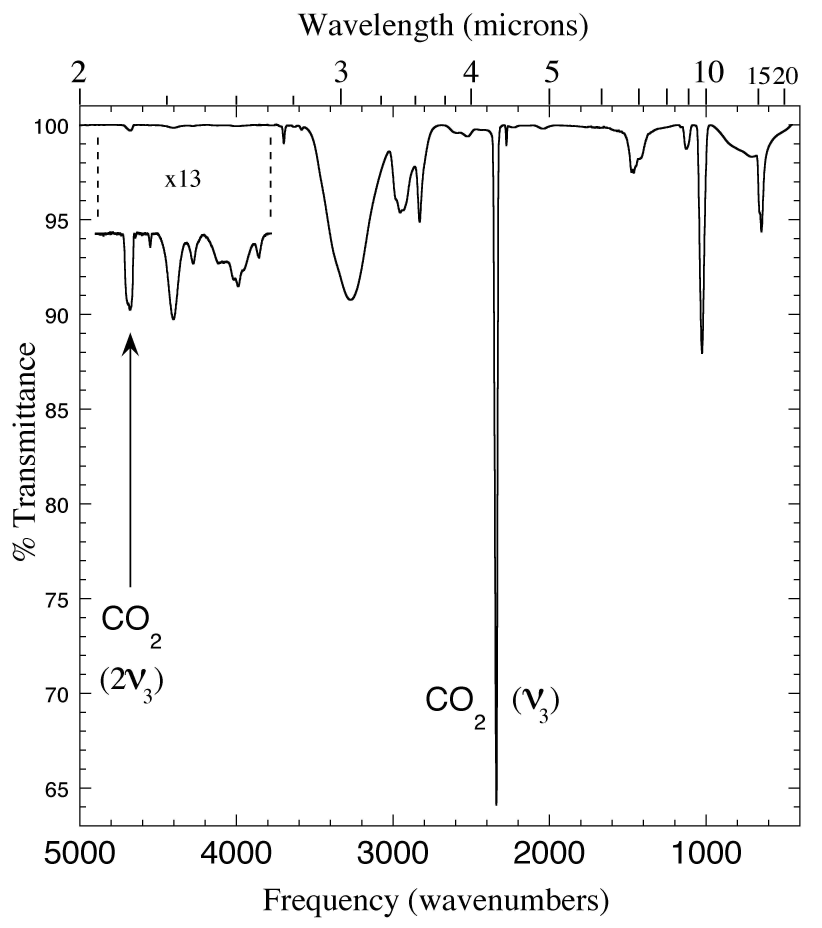
Figure 7. The 2.0-22 micron (5000-450 cm-1) IR spectrum of an CH3OH/CO2 = 5 ice mixture. As in figures 1, 3 and 4, the 2ν3 overtone of CO2 is clearly visible at ~2.14 micron (4682 cm-1), and the pair of CO2 peaks at ~2.70 and 2.79 micron are also present. The other absorptions between 2 and 3 micron are caused by methanol.
Figure 8 (click to enlarge). Temperature dependence of the IR spectrum of a CH3OH/CO2 = 5 ice mixture between 2.041-2.198 micron (4900-4550 cm-1), 2.667-2.817 micron (3750-3550 cm-1), and 4.167-4.386 micron (2400-2280 cm-1). The 2ν3 overtone (left) near 2.13 micron (4700 cm-1) displays multiple components roughly matching those of the ν3 fundamental (right) near 4.27 micron (2340 cm-1). The combination modes (center) near 2.7 and 2.78 micron (3700 and 3600 cm-1) have broad profiles at 15 K that give way on warming to sharper peaks resembling those of pure CO2. However, pure CO2 would have sublimed by 130 K.
These figures are from "Near-infrared laboratory spectra of solid H2O/CO2 and CH3OH/CO2 ice mixtures" published in Icarus, Volume 179, Issue 2, 15 December 2005, Pages 527-534 by Max P. Bernstein, Dale P. Cruikshank and Scott A. Sandford. A PDF version of this paper is available. Please contact us for a copy for the full-text of the article if it is otherwise inaccessible online.
References
Grundy, W. M., Young, L. A., and Young, E. F., "Discovery of CO2 ice and leading-trailing spectral asymmetry on the Uranian satellite Ariel", Icarus, 162, p. 229, 2003
McCord, T. B., et al., "Organics and other molecules in the surfaces of Callisto and Ganymede", Science, 278, p. 271, 1997
McCord, T. B., et al., "Non-water-ice constituents in the surface material of the icy Galilean satellites from the Galileo near-infrared mapping spectrometer investigation", J. Geophys. Res., 103(E4), p. 8603, 1998
Hibbitts, C. A., McCord, T. B., and Hansen, G. B., "Distributions of CO2 and SO2 on the surface of Callisto", J. Geophys. Res., 105(E9), p. 22541, 2000
Hibbitts, C. A., et al., "Carbon dioxide on Ganymede", J. Geophys. Res. (Plan.), 108(E5), p. 5036, 2003
Clark, R. N., et al., "Compositional maps of Saturn's moon Phoebe from imaging spectroscopy", Nature, 435, p. 66, 2005
Buratti, B. J., et al., "Cassini Visual and Infrared Mapping Spectrometer Observations of Iapetus: Detection of CO2", Astrophys. J., 622, p. L149, 2005
Roush, T. L., "Physical state of ices in the outer solar system", J. Geophys. Res., 106(E12), p. 33315, 2001
Hansen, G. B., "The spectral absorption of CO2", Bull. Am. Astron. Soc., 24, p. 978, 1992
Quirico, E. and Schmitt, B., "A Spectroscopic Study of CO Diluted in N2 Ice: Applications for Triton and Pluto", Icarus, 128, p. 181, 1997
Gerakines, P. A., et al., "The Strengths of Near-Infrared Absorption Features Relevant to Interstellar and Planetary Ices", Astrophys. J., 620, p. 1140, 2005
Sandford, S. A. and Allamandola, L. J., "The physical and infrared spectral properties of CO2 in astrophysical ice analogs", Astrophys. J., 355, p. 357, 1990
Dartois, E., et al., "Carbon dioxide-methanol intermolecular complexes in interstellar grain mantles", Astron. & Astrophys., 351, 1066, 1999
Ehrenfreund, P., et al., "Laboratory studies of thermally processed H2O-CH3OH-CO2 ice mixtures and their astrophysical implications", Astron. & Astrophys., 350, p. 240, 1999
Palumbo, M. E. and Baratta, G. A., "Infrared spectra of CO2 in H2O:CH3OH:CO2 icy mixtures", Astron. & Astrophys., 361, p. 298, 2000
|